Atmospheric Disruptions
You recently learned about climate change. This is one major, and important, example of an atmospheric disruption, which is, in simple terms, the things that happen when the atmosphere is thrown off-kilter. The atmosphere is amazing and wonderful and powerful, and she’s a fickle lady prone to throwing temper tantrums. Atmospheric disruption can range from temporary, natural changes like the weather to more permanent, unnatural changes caused by pollution. We’ll talk a little about some examples of each.
The Weather
Ah, the weather. It’s the most boring form of small talk, and also really interesting if you actually know what’s going on. It’s also not exactly “small talk” if the weather decides to take its revenge on the planet in the form of a horrendous hurricane, tumultuous thunderstorm, or terrifying tornado (do you like my onomatopoeia?).
It becomes really important in stormy situations to know about weather patterns so we can see them coming before they happen and evacuate people if needed, or else warn people to take appropriate precautions.
We anticipate that you’re already familiar with standard weather events like wind and rain from your previous schooling. If not, here is a great video on wind and here is great video on rain. We’ll discuss some more extreme weather events here.
It becomes really important in stormy situations to know about weather patterns so we can see them coming before they happen and evacuate people if needed, or else warn people to take appropriate precautions.
We anticipate that you’re already familiar with standard weather events like wind and rain from your previous schooling. If not, here is a great video on wind and here is great video on rain. We’ll discuss some more extreme weather events here.
Thunderstorms
A thunderstorm is any storm that involves the presence of lightning (strikes of light through the sky), which results in thunder (the sound you hear). If you’re unfamiliar with the concept of a thunderstorm or just find them interesting to watch, here’s a video:
It usually rains a lot during a thunderstorm. This is because it takes a while for clouds to be big enough to produce lightning, and this usually goes along with them forming large enough water droplets to rain. Electrical storms without rain can happen, though, and are pretty common in the desert.
Lightning is electrostatic discharge from clouds. Electrostatic discharge is the release of static electricity. You may have felt this if you’ve ever been shocked by a door handle or other bit of metal. If you’re not familiar with that feeling, try putting on a pair of wool socks and shuffling across the carpet: you’re likely to be shocked by the next thing you touch. (Bonus points if you turn the lights off and can see tiny sparks of light when you’re shocked!). Lightning is just this shock on a much, much larger scale.
You see light because you’re charging the gas molecules in the air; you may remember from our chemistry unit that this charged gas is called plasma and is very, very high in energy. You hear thunder because you’re splitting the air: when the two parts of the air come back together, they collide with a large “boom” (or a tiny “pop” in the case of shocking yourself on a bit of metal).
This video gives a great overview of thunderstorms:
Lightning is electrostatic discharge from clouds. Electrostatic discharge is the release of static electricity. You may have felt this if you’ve ever been shocked by a door handle or other bit of metal. If you’re not familiar with that feeling, try putting on a pair of wool socks and shuffling across the carpet: you’re likely to be shocked by the next thing you touch. (Bonus points if you turn the lights off and can see tiny sparks of light when you’re shocked!). Lightning is just this shock on a much, much larger scale.
You see light because you’re charging the gas molecules in the air; you may remember from our chemistry unit that this charged gas is called plasma and is very, very high in energy. You hear thunder because you’re splitting the air: when the two parts of the air come back together, they collide with a large “boom” (or a tiny “pop” in the case of shocking yourself on a bit of metal).
This video gives a great overview of thunderstorms:
Hurricanes
Hurricanes are storms that form over the ocean and have wind speeds of over 74 mph with heavy rain. They often cause severe structural damage because of the wind and flooding from the rain. If you look at them from above, you will see that the clouds make a circular pattern around an “eye.” The eye of the storm is a period of calm followed by more storming.
Hurricanes are storms that form over the ocean and have wind speeds of over 74 mph with heavy rain. They often cause severe structural damage because of the wind and flooding from the rain. If you look at them from above, you will see that the clouds make a circular pattern around an “eye.” The eye of the storm is a period of calm followed by more storming.
Because hurricanes form over the ocean, most of them go away before they ever hit land, so we don’t notice them.
Hurricanes are classified into 5 categories based on their wind speed. 5 is the worst. Below category 1 is considered a tropical storm. Tropical storms are pretty much the same as hurricanes, just milder.
Hurricanes are classified into 5 categories based on their wind speed. 5 is the worst. Below category 1 is considered a tropical storm. Tropical storms are pretty much the same as hurricanes, just milder.
Hurricanes form when the sun heats ocean water to over 82 °F, which evaporates enough water to form lots of huge cumulonimbus clouds over the hotspot. (These are the thunderstorm clouds). Then, upper-level wind (wind high in the atmosphere) and surface wind (wind close to the surface of earth) combine to form the circular pattern. The hurricane can get stronger as it goes. Based on the overall direction of the wind, it may or may not hit land. Once it hits land, it generally starts losing power pretty quickly because it doesn’t have nearly as much water around for the clouds to draw on.
Hurricanes are formed because of hot spots. Global warming makes these hotspots more likely. That means more, bigger hurricanes. Since 2005, the US has faced two of the worst hurricanes ever on record: Katrina (2005), which hit New Orleans and the Gulf, and Sandy (2012), which hit most of the East Coast, including New York City.
This video gives a great overview of hurricanes:
Hurricanes are formed because of hot spots. Global warming makes these hotspots more likely. That means more, bigger hurricanes. Since 2005, the US has faced two of the worst hurricanes ever on record: Katrina (2005), which hit New Orleans and the Gulf, and Sandy (2012), which hit most of the East Coast, including New York City.
This video gives a great overview of hurricanes:
Tornadoes
Tornadoes are rapidly spinning columns of air that touch the ground and come out of cumulonimbus clouds. They come in many shapes and sizes, including:
Tornadoes are rapidly spinning columns of air that touch the ground and come out of cumulonimbus clouds. They come in many shapes and sizes, including:
They create enormous and destructive winds. Usually, wind speeds are less than 110 mph, but they can be over 300 mph. They are ranked from EF0-EF5 (Enhanced Fujita scale) depending on their severity (5 is worst). Here’s a video showing the damage that each of these can cause:
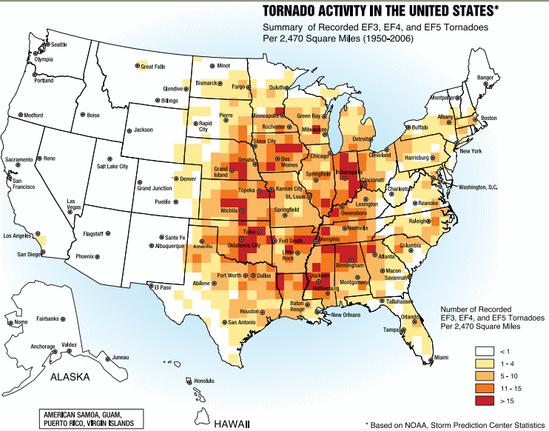
Tornadoes can occur anywhere, but they are most common (by far) in the “Tornado Alley” of the U.S.
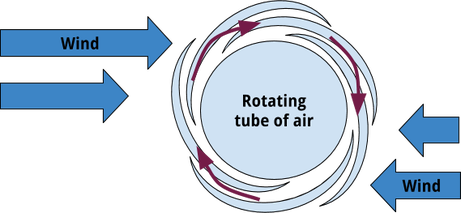
Tornadoes form from supercell thunderstorms, which are huge thunderstorms with rotating upward winds. These storms form when hot, humid air rises from the ground while wind shear forces it in different directions. Wind shear is when air is moving in different directions and at different speeds at different levels of the atmosphere. This creates rotating tubes of air (if you’re having trouble envisioning this, try laying on your side and having someone push the top half of you very quickly one way and pretty slowly the other way. Now you’re rolling on the floor, right?)
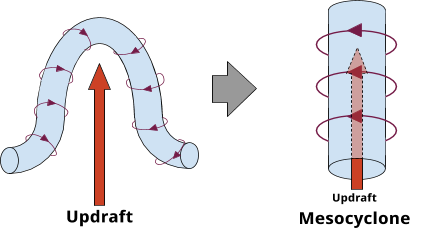
Combined with the updraft, this tube can bend into a horseshoe. Now we have 2 vertical tubes. The counter-clockwise spinning tube wins out (it’s a northern hemisphere thing, like how toilets spin differently in Australia), and we’re left with one vertical, rotating tube. This is called a mesocyclone.
A mesocyclone can grow bigger if there is enough moisture around for the cloud to grow, and continued shear winds will make it spin faster and faster. Eventually, under the right conditions, it could grow into a tornado. It is officially considered a tornado when ground winds around the column reach 40 mph.
This video gives a great overview of tornadoes:
This video gives a great overview of tornadoes:
Because tornadoes result from supercharged thunderstorms, which result from excess heat, humidity, and atmospheric turbulence, global warming also causes tornadoes to be worse and more frequent. This is one of the reasons we most often refer to global warming as climate change—it causes a whole lot more atmospheric destruction than just, “Ooh, it’s warmer today!” implies.
On the opposite end of the spectrum, there’s the weather that makes us happy, energizes us, and makes us long for the moment we can run out of the office or school and soak up the summer sunshine as a light breeze drifts over our faces. In the middle, there’s the weather that may seem sad and nasty, but is actually really important to helping us survive, and eat, and drink clean water (rain). You may love rain. You may not. But you would most definitely miss it if it were gone. So, here’s to the weather! Good, bad, or ugly, you are a fascinating enigma that we want to know more about.
On the opposite end of the spectrum, there’s the weather that makes us happy, energizes us, and makes us long for the moment we can run out of the office or school and soak up the summer sunshine as a light breeze drifts over our faces. In the middle, there’s the weather that may seem sad and nasty, but is actually really important to helping us survive, and eat, and drink clean water (rain). You may love rain. You may not. But you would most definitely miss it if it were gone. So, here’s to the weather! Good, bad, or ugly, you are a fascinating enigma that we want to know more about.
Pollution
In addition to these natural disturbances in our Earth’s amazing atmosphere, there are also some not-so-natural disturbances that affect the conditions of our atmosphere and how we feel because of it. We’ve already talked a lot about one of the most important implications of pollution: climate change. We’ll briefly mention some other ones here, but climate change is the most important for you to understand.
Smog
Smog is visible air pollution. It is a combination of the words “smoke” and “fog”. It is made up of various pollutants, including sulfur oxides, nitrogen oxides, and carbon oxides. These are sometimes called “SOXs,” “NOXs,” and “COXs” because of their chemical formulas, SOₓ, NOₓ, and COₓ, where the X could be any subscript (i.e., COₓ means both CO, carbon monoxide, and CO₂, carbon dioxide). Some pollutants can take on an ugly orange or brown shade in warmer weather, hence why smog is visible. Just because you can’t always see these pollutants doesn’t mean they’re not there.
These pollutants come from coal and fossil fuel burning, car emissions, factory emissions, and forest fires. Note that all of these are caused by humans.
Smog is really bad for your health. It makes it difficult to breathe and can cause the same lung damage as smoking. In fact, when a steel mill in Provo, Utah (a major source of smog) was shut down for a year, local childhood hospitalizations from respiratory disease (like bronchitis, pneumonia, and asthma) were cut in half. The hospitalizations went back up again when the steel mill was reopened. When emissions were especially high, there were even more hospitalizations (Pope III, 1989). This is all good evidence that the steel mill was causing kids to have lung problems.
Smog
Smog is visible air pollution. It is a combination of the words “smoke” and “fog”. It is made up of various pollutants, including sulfur oxides, nitrogen oxides, and carbon oxides. These are sometimes called “SOXs,” “NOXs,” and “COXs” because of their chemical formulas, SOₓ, NOₓ, and COₓ, where the X could be any subscript (i.e., COₓ means both CO, carbon monoxide, and CO₂, carbon dioxide). Some pollutants can take on an ugly orange or brown shade in warmer weather, hence why smog is visible. Just because you can’t always see these pollutants doesn’t mean they’re not there.
These pollutants come from coal and fossil fuel burning, car emissions, factory emissions, and forest fires. Note that all of these are caused by humans.
Smog is really bad for your health. It makes it difficult to breathe and can cause the same lung damage as smoking. In fact, when a steel mill in Provo, Utah (a major source of smog) was shut down for a year, local childhood hospitalizations from respiratory disease (like bronchitis, pneumonia, and asthma) were cut in half. The hospitalizations went back up again when the steel mill was reopened. When emissions were especially high, there were even more hospitalizations (Pope III, 1989). This is all good evidence that the steel mill was causing kids to have lung problems.
Acid Rain
As air pollutants, SOXs, NOXs, and COXs can get trapped in water droplets as they form into clouds and rain. These pollutants are also acidic. Polluted rain is therefore referred to as acid rain.
Plants rely on water from the rain to grow. Acidic water isn’t so friendly to plants: enough acid will kill them. Dead plants means starving animals. As a result of all this, acid rain is destroying many fragile ecosystems, especially rainforests.
Acid rain is also really bad for aquatic environments, because fish, aquatic plants, and other marine life isn’t meant to live in acid. Acidic water is also more likely to dissolve aluminum, which is also toxic to marine life.
Acid rain also hurts us humans. It can hurt crop yield and introduce pollutants into our bodies through the food we eat and the water we drink. Pollutants are bad for our health. We don’t want them in our bodies.
We can stop acid rain by stopping air pollution.
Ozone Depletion
We’ve talked about it before, but as a reminder, the ozone layer protects our earth from harmful UV rays from the sun. It is getting thinner (depleting) due to a specific type of pollutants known as fluorocarbons, which chemically interact with ozone and make it disappear.
Without that crucial protection from the sun, we all get sunburns and skin cancer. Stop it by avoiding products made with fluorocarbons and chlorofluorocarbons (CFCs), like styrofoam.
If you’re interested in learning more about the different kinds of pollution, their negative effects, and what you can do to stop it, this video gives some good detail:
As air pollutants, SOXs, NOXs, and COXs can get trapped in water droplets as they form into clouds and rain. These pollutants are also acidic. Polluted rain is therefore referred to as acid rain.
Plants rely on water from the rain to grow. Acidic water isn’t so friendly to plants: enough acid will kill them. Dead plants means starving animals. As a result of all this, acid rain is destroying many fragile ecosystems, especially rainforests.
Acid rain is also really bad for aquatic environments, because fish, aquatic plants, and other marine life isn’t meant to live in acid. Acidic water is also more likely to dissolve aluminum, which is also toxic to marine life.
Acid rain also hurts us humans. It can hurt crop yield and introduce pollutants into our bodies through the food we eat and the water we drink. Pollutants are bad for our health. We don’t want them in our bodies.
We can stop acid rain by stopping air pollution.
Ozone Depletion
We’ve talked about it before, but as a reminder, the ozone layer protects our earth from harmful UV rays from the sun. It is getting thinner (depleting) due to a specific type of pollutants known as fluorocarbons, which chemically interact with ozone and make it disappear.
Without that crucial protection from the sun, we all get sunburns and skin cancer. Stop it by avoiding products made with fluorocarbons and chlorofluorocarbons (CFCs), like styrofoam.
If you’re interested in learning more about the different kinds of pollution, their negative effects, and what you can do to stop it, this video gives some good detail:
Summary
You should understand:
Be kind to our atmosphere. She might just be more kind to you.
- That thunderstorms form from the combined impact of humidity and unstable pressure systems. Massive clouds acquire an electrostatic charge that is released in the form of plasma (lightning), resulting in the sound of thunder as air is split and brought back together.
- That hurricanes and tornadoes are both extreme, highly destructive examples of thunderstorms.
- Hurricanes form due to hot spots over the ocean, which results in massive cloud production because of all the water available to evaporate.
- Tornadoes form from massive thunderstorms that spin due to wind shear, or wind moving in different directions in different levels of the atmosphere.
- That all extreme weather events are made more frequent and more severe by human-caused climate change.
- That, in addition to climate change, pollution causes a number of negative effects to humans and the environment, including:
- Smog, which causes serious lung problems, including asthma and lung cancer.
- Acid rain, which destroys many fragile ecosystems and threatens agriculture.
- Ozone depletion, which causes increased incidence of skin cancer.
Be kind to our atmosphere. She might just be more kind to you.
Learning Activity
Content contributors: Emma Moulton
Primary literature: Pope 3rd, C. A. (1989). Respiratory disease associated with community air pollution and a steel mill, Utah Valley. American Journal of Public Health, 79(5), 623-628.
Primary literature: Pope 3rd, C. A. (1989). Respiratory disease associated with community air pollution and a steel mill, Utah Valley. American Journal of Public Health, 79(5), 623-628.