Energy Diagrams
A lot of people have difficulty understanding the idea of “energy” because it’s not something that you can see or touch. It isn’t matter. It’s the thing that makes it possible for matter to do stuff. So, don’t feel bad if this idea isn’t quite clicking yet. We’ll try to explain the importance of energy just a little differently, which might be helpful to you. This time, we’ll use handy pictures called energy diagrams.
Potential Energy
The most common types of energy you see in day-to-day life are light, heat, and mechanical energy (like pushing something or fueling a car). In this lesson, we’re especially focused on chemical potential energy. Potential energy, as you might guess from the name, is energy that isn’t doing something yet, but is stored and, if released, would do something. It has the potential to do something. A ball sitting on top of a hill has potential energy: it’s not moving yet, but it has the potential to be moving if someone kicks it. Chemical potential energy is the potential energy stored in chemical bonds. Those bonds haven’t been broken yet, but, if they were, they would release heat or light or power a chemical reaction.
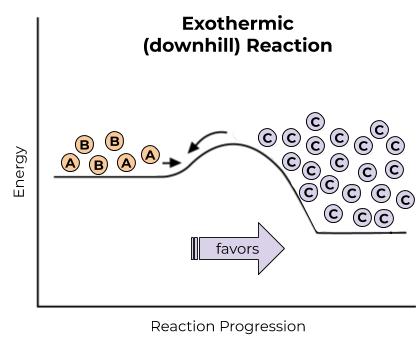
Downhill Reactions
When you’re kicking a ball down a hill, you’re using energy to do work. The work that you are doing is to overcome the attractive forces between the ball and the ground, so that you can move that ball to another place and make new attractive forces between the ball and a different spot on the ground. Kicking the ball takes energy, but, when you kick a ball down a hill, the ball ends up being more stable in the end, because it’s at the bottom of the hill and less likely to be kicked down again. When you’re doing a chemical reaction, you’re overcoming the attractive forces between two atoms so that you can make a new attractive force between different atoms. Breaking the initial bonds takes energy, but, when you power that chemical reaction (give it that initial burst of energy), the ending molecules often end up being more stable in the end, because the bonds are lower in energy, more stable, and less likely to react again.
These downhill reactions are the ones that are going to happen spontaneously. That basically means that they’re going to happen at normal temperatures, and a good amount of product will be made (if we get enough energy input from temperature), because that product is really stable. Remember, a stable molecule is a happy molecule.
Downhill reactions often involve breaking down unstable molecules and making more stable molecules. This often, but not always, means breaking down big molecules and making small molecules.
Downhill reactions often involve breaking down unstable molecules and making more stable molecules. This often, but not always, means breaking down big molecules and making small molecules.
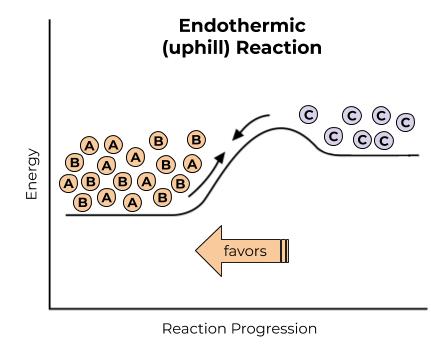
Uphill Reactions
We can also make uphill reactions, like the one shown in the diagram below, happen, it just takes a ton of energy input. Think of trying to kick a ball all the way to the top of a hill: technically possible, it just takes a lot of energy.
Uphill reactions will not happen spontaneously. They need a lot of energy input, for example by heating to really high temperatures, in order to happen. Less product will be made, because the product is less stable than the reactants.
Unfortunately, a lot of really important reactions for life are uphill reactions. Think of making a new protein, for example: sticking a bunch of stable small molecules together and making a big protein, which involves forming a lot of bonds that take a lot of energy to form. Or, think of cell respiration: taking glucose, which stores a lot of energy but is still relatively stable, and making a lot of ATP, which is ridiculously high energy and ridiculously unstable. Even a lot of downhill reactions need a lot of energy input just to get started, even if they will be more stable in the end. Our bodies obviously aren’t heated to crazy temperatures to make these crazy reactions happen, though. So how does life make these unlikely chemical reactions possible?
Unfortunately, a lot of really important reactions for life are uphill reactions. Think of making a new protein, for example: sticking a bunch of stable small molecules together and making a big protein, which involves forming a lot of bonds that take a lot of energy to form. Or, think of cell respiration: taking glucose, which stores a lot of energy but is still relatively stable, and making a lot of ATP, which is ridiculously high energy and ridiculously unstable. Even a lot of downhill reactions need a lot of energy input just to get started, even if they will be more stable in the end. Our bodies obviously aren’t heated to crazy temperatures to make these crazy reactions happen, though. So how does life make these unlikely chemical reactions possible?
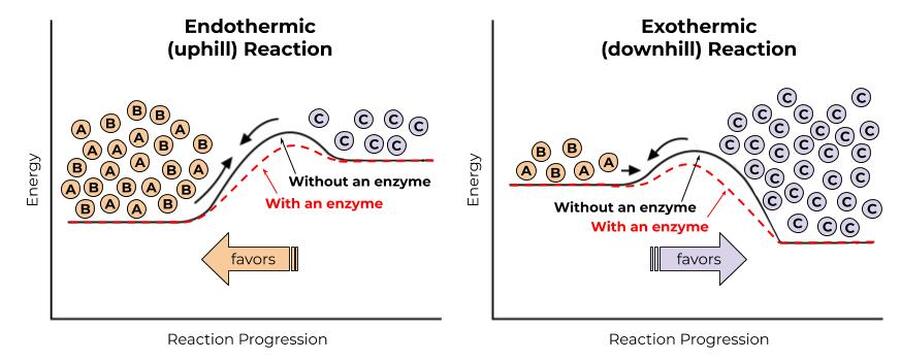
Lifehack 1: Enzymes Lower the Energy Needed For A Reaction
A while back, we mentioned that proteins called enzymes lower the activation energy, or the energy needed to by bringing molecules closer together and in just the right orientation and environment to make chemical reactions possible. It’s kind of like picking up the ball and carrying it to the top of the hill rather than trying to kick it. The process gets easier, but the overall energy change—from the bottom to the top of the hill—doesn’t change.
Enzymes lower activation energy (make “uphills” smaller) by bringing molecules close together in the proper orientation and environment to promote a chemical reaction. Enzymes don’t change which side of the reaction is favored, but they do make it more likely for the reaction to progress.
You can think of enzymes kind of like a molecular matchmaker, or like a meddling friend who is trying to get you to go out with someone specific. She can’t actually change whether or not you two end up liking each other, but she can make sure you two get in the same room together, in a nice romantic environment, while a song that you both just *happen* to really like is playing, thus making it a lot more likely that you two will get together.
Again, enzymes don’t actually change the starting energy or ending energy of the reaction. If we’re going uphill, that’s still not likely to happen all on its own, even with the help of an enzyme. So, we need another way to make reactions “want” to go uphill.
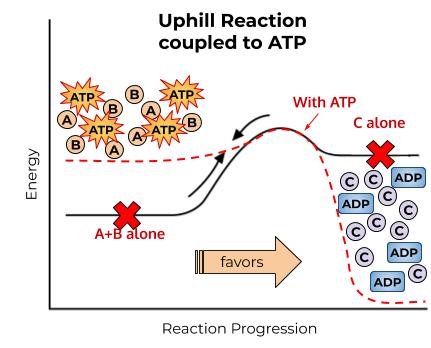
Lifehack 2: Make an Uphill Reaction Downhill with ATP
ATP is a very high-energy molecule. It takes a lot of energy to form, and it’s not very stable. It doesn’t “want” to exist. It “wants” to break into its component molecules, ADP and phosphate. It releases A LOT of energy when it’s broken. It’s like a ball sitting on top of a really, really tall hill. Let’s call that hill Mt. Everest. Once ATP finds something to react with, it will react, and that ball will roll all the way down Mt. Everest.
So, some reactions don’t like to happen all on their own because they have to go up in energy. But, if we throw ATP in the mix, suddenly we can:
So, some reactions don’t like to happen all on their own because they have to go up in energy. But, if we throw ATP in the mix, suddenly we can:
- make some of the reactants higher in energy by putting a phosphate on them
- add in the massive downhill Mt. Everest slide of relaxation and fun times that you get from breaking ATP, and
ATP is high energy and breaking it gives a lot of energy back, so the
overall energy of the products and reactants changes when ATP is involved.
overall energy of the products and reactants changes when ATP is involved.
WEEEEEEEE!!!!!! All the way down the hill.
Also note that ATP can be used to phosphorylate certain proteins, like enzymes or membrane transport pumps, which is a way of turning them on.
Summary
Reaction diagrams are a useful tool for understanding how energy changes during a reaction. You don’t necessarily need to be able to draw them for this class, but it might help you as you’re trying to understand the concepts. You also will likely need to know how to recognize and draw them for later classes.
You should understand:
You should understand:
- That reactions that form complex, high-energy molecules require a significant input of energy and are unlikely to happen on their own, at normal temperatures. This includes many of the reactions of life.
- That enzymes make life possible by lowering the activation energy of a reaction, meaning that they bring molecules together in a way that makes them easier to react.
- That ATP releases a lot of energy when it’s broken, making otherwise unlikely (uphill) reactions happen very readily.
Learning Activity
Content contributors: Emma Moulton