What is Density?
What do you think of when you hear the word “density”?
You may have related density to a lot of things, like size or mass. You may have heard it in context of whether something will sink or float. You may have heard it in context of deciding whether someone is a witch:
You may have related density to a lot of things, like size or mass. You may have heard it in context of whether something will sink or float. You may have heard it in context of deciding whether someone is a witch:
Or maybe you have never tried to sink a witch. I mean, maybe.
Maybe the word density is completely new to you. Density is an important concept in chemistry because it helps us to characterize things. For example, diamond and cubic zirconia look very similar on the outside. But, diamond is very expensive while cubic zirconia is relatively cheap. If you wanted to tell them apart, though (for example, to make sure someone isn’t trying to sell you a fake diamond instead of a real one), all you would have to do is take a look at the density: Cubic zirconia is about twice as dense as diamond.
Maybe the word density is completely new to you. Density is an important concept in chemistry because it helps us to characterize things. For example, diamond and cubic zirconia look very similar on the outside. But, diamond is very expensive while cubic zirconia is relatively cheap. If you wanted to tell them apart, though (for example, to make sure someone isn’t trying to sell you a fake diamond instead of a real one), all you would have to do is take a look at the density: Cubic zirconia is about twice as dense as diamond.
Let’s look at another example that you may be familiar with. Which weighs more, a pound of feathers or a pound of bricks?
Gotcha! (Or maybe not). A pound of feathers will weigh exactly the same as a pound of bricks (and have the same mass). Why? Because a pound is a measure of weight. You will just need a lot more feathers to make up a pound. In other words, you need a larger volume. So, which is more dense?
Gotcha! (Or maybe not). A pound of feathers will weigh exactly the same as a pound of bricks (and have the same mass). Why? Because a pound is a measure of weight. You will just need a lot more feathers to make up a pound. In other words, you need a larger volume. So, which is more dense?
That little one? About 2 pounds of brick. That giant trashbag? Not even a pound of feathers. (It’s ¾ of a pound, if you must know.)
The bricks! It packs that 1 pound’s worth of matter in much more compactly--it has “more bang for your buck,” so to speak.
This measure of how much weight we can pack into a given volume is called density. Density is basically how compact or “crowded” something is--how much matter we have squeezed into a certain amount of space. Mathematically, it is defined as the mass (or weight) per volume:
D = m/v
Consider a subway station during morning rush hour when there are people everywhere. Now consider the same subway station in the middle of the day, when there are only a few people in the subway station. The volume, or size of station, stays the same, but the number of people--how crowded the station is--has gone down.
Since there are fewer people during the middle of the day, the “density” of the station is lower. This video does a good job of explaining the basic concept of density:
Since there are fewer people during the middle of the day, the “density” of the station is lower. This video does a good job of explaining the basic concept of density:
Sink or Swim?
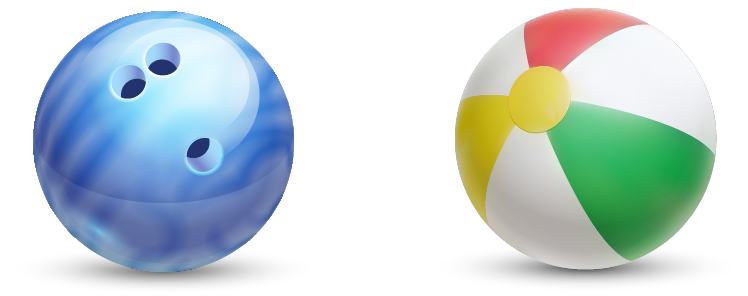
Now, consider another example. Which is more dense, a bowling ball or a beach ball?
You know that a bowling ball weighs more than a beach ball. This means that it also has a larger mass. Mass and weight aren’t exactly the same thing, but the difference isn’t really important unless you’re space travelling. So, the bowling ball has a larger mass, but is about the same size as a beach ball. Based on what you know so far, which one has the higher density?
You know that a bowling ball weighs more than a beach ball. This means that it also has a larger mass. Mass and weight aren’t exactly the same thing, but the difference isn’t really important unless you’re space travelling. So, the bowling ball has a larger mass, but is about the same size as a beach ball. Based on what you know so far, which one has the higher density?
|
Bowling Ball
|
Beach Ball
|
If you said the bowling ball, you’ve got the idea! In order to have a larger mass in the same amount of volume, we must have squished more molecules into the same amount of space. You’ll note that, the denser something is, the more likely it is to sink in water. The rule is that a denser thing will sink in a less dense thing. A solid bowling ball has molecules that are packed more tightly than water, so it will sink, while a beach ball full of air has molecules that are far apart and much less dense than those in water, so it will float.
To reiterate: If an object is denser than water, it sinks. If it is less dense, it floats. The same idea goes for all liquids: If something is more dense than oil, it sinks (in oil). If something is less dense than oil, it floats on oil. You will get more practice with this concept when you do the EIS.
To reiterate: If an object is denser than water, it sinks. If it is less dense, it floats. The same idea goes for all liquids: If something is more dense than oil, it sinks (in oil). If something is less dense than oil, it floats on oil. You will get more practice with this concept when you do the EIS.
Density of Solids, Liquids, and Gases
On this topic, we’ve just learned about the states of matter. Based on what you know about solids, liquids, and gases, what do you know about their densities?
If you guessed that solids are most dense, liquids are medium-dense, and gases are least dense, you understand this concept well! Most solid objects are denser than liquids, because the particles in solids are packed more tightly together. Good job.
This video does a good job of explaining the density of various states of matter:
If you guessed that solids are most dense, liquids are medium-dense, and gases are least dense, you understand this concept well! Most solid objects are denser than liquids, because the particles in solids are packed more tightly together. Good job.
This video does a good job of explaining the density of various states of matter:
Note, however, that there are exceptions to this rule. For example, you may note that some solid objects like wood tend to float on water. You may have also noticed marshmallows floating on hot chocolate, or a beach ball floating on water. Almost all exceptions like this are because that solid object actually contains a lot of air, which is not dense. Wood contains a lot of spaces and cavities that fill with air. Marshmallows are pumped full of air, which allows them to float. A beach ball has only a thin solid membrane, and mostly air. A bowling ball, which is solid throughout, will, however, sink in water.
Density of Solid and Liquid Water
Another very important exception to the rule of “solids are denser than liquids” is water. When drinking a cold glass of water, have you ever noticed that the ice floats? This is because water’s solid form is actually less dense than its liquid form.
This is because water is held together by hydrogen bonds. In liquid water, these bonds are fluid and flexible, and can slide past each other easily. You can think of this like a crowded airport where everyone is getting around pretty quickly. In solid water, you still have hydrogen bonds, but you have a lot less kinetic energy and “slide-y-ness”. Instead, the hydrogen bonds act like spacers holding the molecules apart, because molecules aren’t moving fast enough to challenge the elasticity of these bonds. You can imagine this like the same crowded airport, but everyone is holding a ten-foot pole to try to keep as far apart from other people as possible. So, in this case, less movement means less density. This video does a good job of explaining this idea:
This is because water is held together by hydrogen bonds. In liquid water, these bonds are fluid and flexible, and can slide past each other easily. You can think of this like a crowded airport where everyone is getting around pretty quickly. In solid water, you still have hydrogen bonds, but you have a lot less kinetic energy and “slide-y-ness”. Instead, the hydrogen bonds act like spacers holding the molecules apart, because molecules aren’t moving fast enough to challenge the elasticity of these bonds. You can imagine this like the same crowded airport, but everyone is holding a ten-foot pole to try to keep as far apart from other people as possible. So, in this case, less movement means less density. This video does a good job of explaining this idea:
This turns out to have important implications for life on earth, especially marine life. In this way, ice can float on water, allowing all the fishes and other life to keep thriving and keep insulated under the surface. We’ll talk more about how the special properties of water are beneficial to life later on.
Again, remember that water is an exception. In general, the bonds in solids hold them together very compactly, making most solids more dense than liquids, where the bonds do not hold the molecules as close together.
Summary
You should understand:
- The basic definition of density, which is the amount of mass (weight) we can fit into a certain volume. Molecules are more crowded in more dense objects. Molecules are less crowded in less dense objects.
- How the concept of density relates to whether something will sink or float.
- How the density of solids, liquids, and gases relate to each other both as a general rule and in the exception case, water.
Learning Activity
Content contributors: Emma Moulton and Emily Zhang