Differentiation
Particle sorting: we’ve talked about it before, we just haven’t put a name to it yet. Quite simply, particle sorting describes how “stuff” (in this lesson, we’ll mainly talk about rocks) are separated based on their density and size. This process is known also known as differentiation.
If you can follow, this guy will shout basically everything you need to know about particle sorting:
If you can follow, this guy will shout basically everything you need to know about particle sorting:
Particle sorting/differentiation describes how quickly things will sink in still water. It can be a bit tricky to wrap your mind around because it seems to contradict itself. These are the two main rules that dictate how it occurs:
- Dense stuff sinks.
- Small stuff sinks.
We’ll go through each of these to explain where this contradiction comes from, and we’ll then look at the example of gold panning to see how each becomes important in a real-world context.
Dense Stuff Sinks
If we consider these rules separately, both make perfect sense. We know that heavy stuff sinks because we can see it happen. We can see a rock sink in water. We can see oil float on water. We also know that the layers of the Earth are the way they are because the densest stuff (iron) sank to the middle. In fact, if we could somehow magically make all particles the same size, we would see the heavy stuff settle to the bottom every time. For example, if we put baseballs in a ballpit and shook it around a bit, we would quickly see the baseballs settle to the bottom, like so:
This is especially true in water and other liquids (versus gases/air), where the buoyant force is more significant and the difference between how lighter things and heavier things get affected by that force is greater. But, the same still holds true as long as a buoyant force exists. We’re not super worried about you understanding the precise physics of this for this class, but, if you’d like a little more explanation, this video gives a great overview of buoyancy:
We’re mainly interested in the takeaway here, which should be pretty obvious to you based on what you learned about density in Chemistry: Denser things sink faster than less dense things.
Small Stuff Sinks
But, that’s not the full story, because we also know that small stuff sinks. For example, if we put sand in a jar of rocks, the sand would fall in between the cracks to the bottom. Or, if we put salt on popcorn, the salt will fall to the bottom, and we still have bland popcorn. If we completely control for density (make everything the same), this will always be the case: the small stuff will sink.
Separately, those two ideas should be pretty easy to wrap your head around. When we put them together, though, things might get a bit confusing. After all, we usually associate the heaviest stuff with the biggest stuff. But, remember, this is about density, not weight. And density is weight (mass) divided by volume: it takes size into account. So, something “light” could actually be quite dense, if it’s small. Be sure you understand the difference between density and mass.
Even if you do understand this idea, dense stuff can be big and non-dense stuff can be small. The real world isn’t a perfect system like our examples were. That can happen. So, let’s look at a few real world examples to understand particle sorting better.
Even if you do understand this idea, dense stuff can be big and non-dense stuff can be small. The real world isn’t a perfect system like our examples were. That can happen. So, let’s look at a few real world examples to understand particle sorting better.
Panning for Gold
Let’s start with a little history lesson. In 1849, courageous (or, if you prefer, foolish) Americans “rushed” to California in a maniacal gold craze: the Gold Rush of 1849.
Very few would be successful, but these guys are clearly super excited about their panning (okay, you can’t really tell because everything is black or white and their mustaches cover their mouths, but they are SO excited).
The pans the men are holding show you how particle sorting works in real life. To find small deposits of gold in the river, the guys would scoop a pan full of sediment, then shake the pan until, magically, the only particles left over were gold particles. They would usually only find tiny bits of gold dust, but, just like a drop of water may lead back to the Niagara, these little bits could lead them to the “gold vein,” and they would make it RAIN!
Watch this guy explain how to pan for gold:
The pans the men are holding show you how particle sorting works in real life. To find small deposits of gold in the river, the guys would scoop a pan full of sediment, then shake the pan until, magically, the only particles left over were gold particles. They would usually only find tiny bits of gold dust, but, just like a drop of water may lead back to the Niagara, these little bits could lead them to the “gold vein,” and they would make it RAIN!
Watch this guy explain how to pan for gold:
I’ll go into a little more detail now.
You know that items denser than water will sink and items less dense than water will float. Every particle in sediment (that’s a fancy word for dirt and rocks) is denser than water. But, they’re not necessarily the same density as each other. Gold, for example, is denser than most rocks. When we sort particles, we take advantage of their density and basically make them race to the bottom of the pan whenever we shake it. Let’s take a look.
You know that items denser than water will sink and items less dense than water will float. Every particle in sediment (that’s a fancy word for dirt and rocks) is denser than water. But, they’re not necessarily the same density as each other. Gold, for example, is denser than most rocks. When we sort particles, we take advantage of their density and basically make them race to the bottom of the pan whenever we shake it. Let’s take a look.
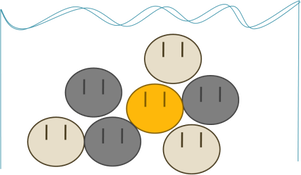
Here are some particles in water. Gold is colored in gold, because gold is gold and that’s the goldiest of the golds. Right now, they’re arranged randomly, just sitting around until we shake the pan. To see what happens when we shake the pan, let’s pretend like they all had a fair start, like so:
Based on what you know about particle sorting already, can you guess what happens next?
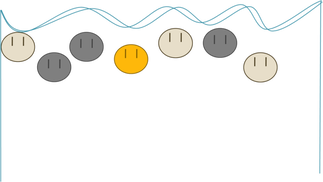
You guessed it! (Probably). Gold, with the greatest density, falls the fastest.
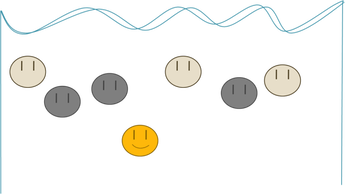
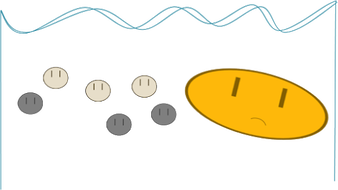
Now imagine that the gold was really big and everything else was tiny:
Now imagine that the gold was really big and everything else was tiny:
This time, the gold is fatter, and therefore slower (remember that small stuff sinks faster). There are two reasons that gold might not “get the gold” in this race:
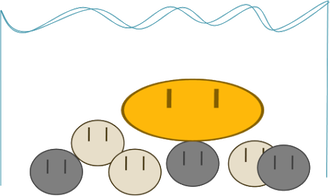
Let’s see what happens when gold, the dense stuff, is REALLY BIG and the other stuff is REALLY SMALL.
- Other particles might get in its way, because it is so big.
- It might get slowed down by that buoyant force thingy I mentioned. (Don’t worry too much about the details, but I’ll tell you anyways: buoyant force pushes up on stuff based on its volume. Bigger stuff gets pushed up more by water. That’s why ships can float even though they’re made of metal.)
Let’s see what happens when gold, the dense stuff, is REALLY BIG and the other stuff is REALLY SMALL.
Buoyant force pushes up on the gold SO MUCH because it is SO BIG that it doesn’t win the race to the bottom. In terms of gold panning, this isn’t really a big deal, because, if the gold were that big, the panner would just be able to pull it out and sell it for lots of money.
We could calculate the exact size that this gold would have to be, based on its density and the size and density of the other particles in suspension, for it to lose this race. In other words, we could determine exactly how big our gold fragment had to be before panning would no longer be useful. But, that’s some pretty complicated math, which is a little above your current learning level, so I’ll let you learn about that when you’re older, smarter, and know more about Newtonian physics.
We could calculate the exact size that this gold would have to be, based on its density and the size and density of the other particles in suspension, for it to lose this race. In other words, we could determine exactly how big our gold fragment had to be before panning would no longer be useful. But, that’s some pretty complicated math, which is a little above your current learning level, so I’ll let you learn about that when you’re older, smarter, and know more about Newtonian physics.
Summary
You should understand:
- That dense stuff sinks.
- That small stuff sinks.
- That these two rules sometimes contradict each other, in which case we’d have to do an experiment (or do math, which we won’t in this course) to figure out which actually occurs.
Learning Activity
Content contributors: Emma Moulton and Emily Zhang