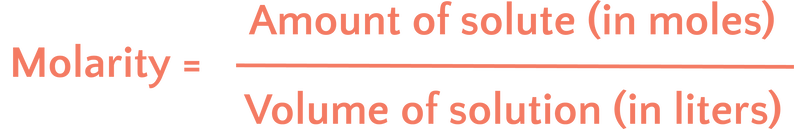Solutions and Molarity
Before we can move on and talk more about diffusion, you must first understand the concept of molarity. In order to understand this, you first need to know what a solution is. These concepts might be new to you, but they’re really pretty simple.
What is a Solution?
No, we don’t mean “solution” like “answer” here. The answer to “What is a solution?” in chemistry, in the simplest possible terms, is that a solution is stuff dissolved in stuff. More specifically, it is a solute (such as salt, sugar, or whatever) dissolved in a solvent (such as water).
For a fun (and tasty!) activity that will help you understand what a solution is, try making some lemonade! It is for science, after all. Here is a recipe:
You could also do this with chocolate milk or many other kitchen ingredients. Cooking is chemistry!
As you mix your lemonade, think: What is/are the solute(s) and what is/are the solvent(s)?
If you’re still having trouble with distinguishing a solvent from a solute, this video explains it well:
For a fun (and tasty!) activity that will help you understand what a solution is, try making some lemonade! It is for science, after all. Here is a recipe:
- 2 quarts of water
- 1 cup lemon juice
- 1 cup sugar
You could also do this with chocolate milk or many other kitchen ingredients. Cooking is chemistry!
As you mix your lemonade, think: What is/are the solute(s) and what is/are the solvent(s)?
If you’re still having trouble with distinguishing a solvent from a solute, this video explains it well:
When a solute is dissolved into a solvent, its particles spread out evenly in the solvent. In other words, they diffuse outward in solution, down their concentration gradient, until they reach equilibrium. So, in lemonade, the molecules of sugar diffuse through the water/lemon juice until the entire solution is equally sugary. Yum! (If you’re shaking or stirring, there’s also some dispersion going on here). Diffusion creates solutions.
Note the use of the word dissolved when we talk about solutions. That means that the solute is mixed into the solution on a molecular level. Even if we looked at it under a microscope, we wouldn’t be able to distinguish the solute and the solvent. This is different from a mixture, which isn’t as well mixed. This video does a good job of explaining the difference between a solution and a mixture:
What is Molarity?
Now let's move onto molarity. Molarity is a measure of how concentrated a solution is. Concentrated means “crowded,” or how much solute there is. Concentration/molarity is the ratio of solute to solvent. For example, if you added more lemon juice to your lemonade, we could say that the concentration of lemon juice is higher. If we doubled the sugar, we could say that we doubled the molarity of sugar.
What is useful about molarity is that it is quantitative. Things have numbers instead of just being “pretty concentrated” or “pretty dilute” (dilute is the opposite of concentrated, so there is relatively little solute—like in really weak lemonade). We can calculate molarity with the following equation:
What is useful about molarity is that it is quantitative. Things have numbers instead of just being “pretty concentrated” or “pretty dilute” (dilute is the opposite of concentrated, so there is relatively little solute—like in really weak lemonade). We can calculate molarity with the following equation:
Or more simply:
Now, at this point, you may be thinking, “Whoa, whoa, whoa… what is this ‘mol’ thing?” Well, it is the abbreviation for mole, which is both a very important measurement in chemistry and a funny looking animal:
We’ll focus on the chemistry definition, although I’m sure we could take a really fascinating deep dive on the animal if we wanted. A mole is a unit of measurement, like “hundred” or “dozen”. A hundred eggs is 100 eggs. A dozen doughnuts is 12 doughnuts. Two dozen doughnuts is 24 doughnuts. A mole of water is 6 x 10²³ water molecules. That escalated quickly.
Just think about how big this number is for a second. 6 x 10²³! In one mole! That means that 18 mL of water (that’s less than 1/10 of a cup) has 600,000,000,000,000,000,000,000 (600 MILLION MILLION BILLION!) water molecules. Wowza!
One mole of anything is 6 x 10²³ units of that thing. 6 x 10²³ cupcakes is one mole of cupcakes. 6 x 10²³ doughnuts is one mole of doughnuts. Two moles of water is 12 x 10²³ water molecules. Three moles of eggs is 18 x 10²³ eggs, and so on. A mole of any substance is 6 x 1023 individual units of that substance. This number is called Avagadro’s number, and it is the most common way that you will see scientists “count” individual atoms or molecules.
Flashback and retcon, for your general information: The atomic masses that you see on the periodic table, measured in amu, are the same as grams/mol. That’s how Avagadro decided on the number for mol. So, there is 1 g of hydrogen atoms per mole of hydrogen atoms, 12 g of carbon atoms per mole of carbon atoms, and so on.
This video does a good job of explaining what moles are:
Just think about how big this number is for a second. 6 x 10²³! In one mole! That means that 18 mL of water (that’s less than 1/10 of a cup) has 600,000,000,000,000,000,000,000 (600 MILLION MILLION BILLION!) water molecules. Wowza!
One mole of anything is 6 x 10²³ units of that thing. 6 x 10²³ cupcakes is one mole of cupcakes. 6 x 10²³ doughnuts is one mole of doughnuts. Two moles of water is 12 x 10²³ water molecules. Three moles of eggs is 18 x 10²³ eggs, and so on. A mole of any substance is 6 x 1023 individual units of that substance. This number is called Avagadro’s number, and it is the most common way that you will see scientists “count” individual atoms or molecules.
Flashback and retcon, for your general information: The atomic masses that you see on the periodic table, measured in amu, are the same as grams/mol. That’s how Avagadro decided on the number for mol. So, there is 1 g of hydrogen atoms per mole of hydrogen atoms, 12 g of carbon atoms per mole of carbon atoms, and so on.
This video does a good job of explaining what moles are:
At this point, you may be asking, “Okay, okay. So moles is just a measure of how many molecules there are. What is L?”
You may be more familiar with this one: A liter (abbreviated L) is also a unit of measurement. If you’ve ever seen a large bottle of soda at a party, that bottle contains 2 liters of soda. 1 liter is about equal to 1 quart. (Note: you may also see liter spelled “litre”. This is the British spelling).
Some Calculations
Now that we’ve addressed the parts of molarity, we can combine them. Reference the above equations.
A solution contains 1 liter of water and 3 moles of salt. What is the molarity of the solution?
Because we know the number of moles, 3, and we know that there is 1 liter of water, we do simple math: 3/1 = 3. Thus, the molarity (M) is 3 M.
A solution contains 2 liters of water and 4 moles of salt. What is the molarity?
We have 4 moles of salt, and 2 liters of water. Because we need to know the number of moles per liter, we simply divide them both by 2 (or simply divide them normally). Thus, 4/2 = 2, so the molarity of the solution is 2 M.
A solution contains 1 liter of water and 3 moles of salt. What is the molarity of the solution?
Because we know the number of moles, 3, and we know that there is 1 liter of water, we do simple math: 3/1 = 3. Thus, the molarity (M) is 3 M.
A solution contains 2 liters of water and 4 moles of salt. What is the molarity?
We have 4 moles of salt, and 2 liters of water. Because we need to know the number of moles per liter, we simply divide them both by 2 (or simply divide them normally). Thus, 4/2 = 2, so the molarity of the solution is 2 M.
Summary
You should understand:
- What a solution is and how it is different from a mixture.
- What a solute and solvent are.
- What a mole is and how it relates to molarity.
- How we measure concentration using molarity.
- How to calculate molarity from a given number of moles and volume.
Learning Activity
Content contributors: Connie Chen, Emma Moulton