Rocks and Minerals
Rocks. They’re everywhere. They’re gorgeous. And they are the subject of this lesson. Before we get into the details, just enjoy this beautiful ode to the majesty of rocks:
#GetOutInNature
Rocks and minerals are found all around us, in the land beneath our feet, the countertops in our kitchen, and even in the hardware of our electronics. They have a significant influence on our lives, but how much do we really know about them?
Rocks and minerals are found all around us, in the land beneath our feet, the countertops in our kitchen, and even in the hardware of our electronics. They have a significant influence on our lives, but how much do we really know about them?
What are Minerals?
Let’s begin by clarifying something: Rocks and minerals are not the same thing. Minerals are naturally occurring crystalline structures of inorganic compounds. Each of these crystals is made up of a single, pure substance—often ionic compounds, and sometimes covalent molecules—and therefore have distinct physical and chemical properties depending on the exact compound or molecule that makes them up.
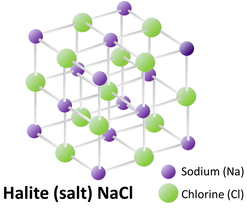
One example of a common mineral is halite, which is just the crystalline structure of NaCl—table salt.
One example of a common mineral is halite, which is just the crystalline structure of NaCl—table salt.
The Salt Flats in Utah are made up mainly of halite:
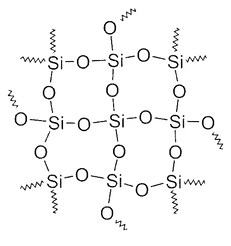
Another common mineral is quartz, which is the crystalline structure of silicon dioxide (SiO₂):
What Are Rocks?
Rocks, on the other hand, are formed from combinations of minerals and organic compounds. Although there are certain classifications of rock that tend to contain similar substances, the exact composition of the rock and the proportions of the various substances that it contains will vary based on where and how that rock formed.
Granite, for instance, contains between 10 and 50 percent quartz and can sometimes include mica and hornblende.
Granite, for instance, contains between 10 and 50 percent quartz and can sometimes include mica and hornblende.
Granite, which is often used in countertops, contains different amounts of minerals depending on where it formed.
El Capitan-- one of nature's largest granite monolith's at about 3,000 feet tall is both beautiful and a popular objective for rock climbers.
How do We Classify Rocks?
Since we can’t define the chemical nature of a rock exactly, like we can describe minerals by their chemical structures, scientists have turned to classification to make understanding the composition of rocks easier. There are 3 main classes of rocks, which are defined by how they’re formed: igneous, sedimentary, and metamorphic. We can also put rocks into more specific categories like “granite,” which is more closely related to the composition of the rock, but we won’t worry about getting that detailed in this class.
Igneous rocks form from magma bubbled up from the mantle below Earth’s crust, and then cooled. Rapid cooling results in many small crystals that cannot be seen with the naked eye, so the rock looks and feels smooth. Obsidian, shown below, is a good example of an igneous rock.
Igneous rocks form from magma bubbled up from the mantle below Earth’s crust, and then cooled. Rapid cooling results in many small crystals that cannot be seen with the naked eye, so the rock looks and feels smooth. Obsidian, shown below, is a good example of an igneous rock.
Black obsidian, an igneous rock
Gas bubbles can also be trapped in magma during cooling (remember that carbon dioxide is formed from the recycling of old, organic crust). Pumice, another type of igneous rock, gets its porous appearance from these gas bubbles. Because of its structure, pumice is less dense than water and floats in the liquid.
Pumice, an igneous rock with trapped gas bubbles.
Sedimentary rocks form from, as the name suggests, sediments. Sediment is basically a fancy word for dirt: A mixture of ground up old rock and decaying organic material. Sedimentary rocks can be further classified based on their composition. Clastic sedimentary rocks are formed from the mechanical weathering debris while chemical sedimentary rocks are formed from precipitates of dissolved materials. These rocks are often easy to spot because of their “chunkiness,” meaning that you can actually see the broken up old chunks of rocks and minerals, as in granite (shown above) or hematite:
Hematite, a sedimentary rock rich in iron
Sometimes, sedimentary rocks are made up of smaller sediment particles that appear deceptively smooth, making these sedimentary rocks more difficult to spot. One example is shale:
Shale forms from silt, clay, and mud, so it has a smooth appearance, unlike many other sedimentary rocks.
Metamorphic rocks are rocks that have been changed (hence, metamorphic, like how a caterpillar changes into a butterfly by metamorphosis) by intense heat and pressure. These conditions are often found deep underground. Rocks can also be changed by chemical processes. Any rock can become a metamorphic rock given enough heat and pressure, including sedimentary, igneous, and other, older, metamorphic rocks.
Metamorphic rocks can typically be recognized because of their banding pattern, which forms as a result of different layers of rock being forced together under pressure:
These are good classifications to understand. But, they aren’t fixed. Just like any rock can become metamorphic under enough heat and pressure, rocks can become sediment if they are crushed enough and, eventually, igneous as Earth’s crust becomes recycled due to plate tectonics. This cheesy video will explain more:
Cool, right? Which brings us back to a couple of important ideas that we’ve brought up before: First, that categories aren’t always perfect, and the real world is often more complex than our simple categories account for. Second, even things that seem fixed, like rocks, or the surface of the earth, are constantly changing. They’re just doing it slowly.
Density of Rocks and Minerals
The density of various rocks and minerals is dependent on their composition and formation. It can be hard to predict the density of a rock even if you know what type it is. This is because different amounts of minerals can go into it depending on where it was formed, and each of these minerals has different densities. Also, like with pumice, gas bubbles can get trapped in the rock, giving them very low density. For this reason, the most reliable way to determine the density of a specific rock is to measure it. Just like we did in chemistry, we can measure the volume of the rock by fluid displacement (sinking it in water and seeing how much the volume changes) and the mass of the rock using a scale. We can then calculate density with the equation d = m/V.
In the case of minerals, specific gravity is a useful feature for identification, as each mineral has a fairly consistent density. The specific gravity of something is just the density of one thing relative to the density of another thing (a ratio). Commonly, we use the specific gravity relative to water, which has a density of 1 g/mL. In this case, specific gravity is the exact same as density (because we’re just dividing by 1). Saying that specific gravity is helpful for identifying minerals is very similar to saying that density is helpful for identifying unknown metals, like we practiced in the Chemistry unit. This video demonstrates the usefulness of specific gravity for identifying minerals well:
In the case of minerals, specific gravity is a useful feature for identification, as each mineral has a fairly consistent density. The specific gravity of something is just the density of one thing relative to the density of another thing (a ratio). Commonly, we use the specific gravity relative to water, which has a density of 1 g/mL. In this case, specific gravity is the exact same as density (because we’re just dividing by 1). Saying that specific gravity is helpful for identifying minerals is very similar to saying that density is helpful for identifying unknown metals, like we practiced in the Chemistry unit. This video demonstrates the usefulness of specific gravity for identifying minerals well:
Metallic minerals generally have higher specific gravities than non-metallic minerals, as they contain heavier metal elements and are held together by stronger intermolecular attractions: ionic bonds. Non-metallic minerals are held together by the relatively weaker coordinate covalent bonds, which are still strong, but not quite as strong. So, molecules are pulled tighter together in metallic minerals, making them more dense.
It’s harder to come up with a general trend for the density of rocks, because there are a lot of exceptions. But, in general, sedimentary rocks tend to be the least dense, because they are basically loosely packed dirt, igneous rocks are of a medium density, and metamorphic rocks are of the highest density because the contents have been pushed together under pressure. This should make sense based on what you have learned about how different rocks are formed. You should also recognize that there are many exceptions, such as pumice being a very lightweight rock due to the trapped gases.
Summary
You should understand:
- The difference between rocks and minerals.
- The difference between igneous, sedimentary, and metamorphic rocks in terms of their general appearance and how they are formed.
- How to calculate density of a rock or mineral.
- How to identify an unknown mineral from its specific gravity and a chart of known specific gravities.
- The general trends in density of rocks and minerals:
- Metallic minerals are generally more dense than nonmetallic minerals.
- With many exceptions, metamorphic rocks are generally more dense than igneous rocks, which are generally more dense than sedimentary rocks.
Learning Activity
Content contributors: Suzanne Xu, Emma Moulton