States of Matter
In this lesson, you will learn about the states of matter. As you’ve learned, matter is stuff. It is you, me, and everything else in the universe. And, like a shapeshifting alien or a Transformer, matter can change forms. These forms are called states, as in “a way of being.” You can imagine matter getting very introspective and asking itself, “What am I? Why am I the way I am? How do I change? How do I become?” In this lesson, we will journey inward with matter on it’s introspective journey of self-discovery as we answer these important questions.
The States of Matter
One day the air looked to the ocean. He thought, “Wow, I wonder what it takes to become so vast, yet so powerful and fluid.” Then he thought, “All I can do is make wind.” And, as he started to make wind, the ocean rose and crashed its waves and thrashed every which way, all the while wondering, “I wonder what it’s like to be air, so light, so unassuming and spacious, and yet so strong and capable of causing me so much change without even realizing it.” But the air didn’t see what it was doing to the ocean. All it saw was waves dancing and frolicking and having a good time—which just made the air want to be ocean more. The air wanted to be ocean so badly, and it couldn’t understand why it was that they were so different anyhow. The air started to cry. And as air cried, its tears became ocean.
What is the point of this story? Well, mostly it’s just nice poetry, and maybe it’s supposed to teach you something about not comparing yourself to others (I mean, maybe). It also goes to show you that all matter can be found in different states, or ways of being, that these states have different properties, and that matter can change between states. Bye-bye emotion story, it’s science time.
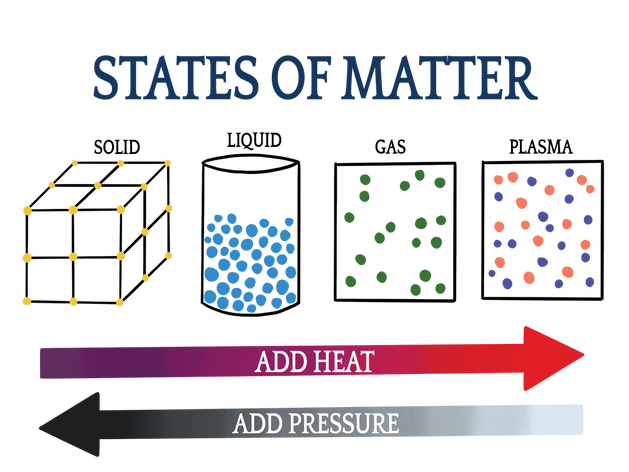
The three common states of matter are solid, liquid, and gas. A fourth state is plasma, which we’ll talk a bit about because it’s cool, but won’t worry too much about for this class.
What is the point of this story? Well, mostly it’s just nice poetry, and maybe it’s supposed to teach you something about not comparing yourself to others (I mean, maybe). It also goes to show you that all matter can be found in different states, or ways of being, that these states have different properties, and that matter can change between states. Bye-bye emotion story, it’s science time.
The three common states of matter are solid, liquid, and gas. A fourth state is plasma, which we’ll talk a bit about because it’s cool, but won’t worry too much about for this class.
Solids
The way molecules are structured is very important in determining the state an object will be in. In a solid, molecules are bonded into very compact departments. Under this umbrella, there are two main configurations atoms can have. The first is called a geometric lattice. In a geometric lattice, atoms are well organized into a specific type of structure. Most solids are found in a geometric lattice. Solids in a geometric lattice are called crystalline solids; examples include metals, ionic compounds, minerals (like diamonds), and ice cubes.
Not all solids are organized into a geometric lattice. Some solids are considered amorphous. Amorphous solids aren’t nicely structured like crystals; they’re more like a bunch of molecules just got smushed together. Examples include glass, gels, thin films, and nanostructures.
Seeing what a broad range of structures are encompassed by the term "solid," it may seem like a bit of a challenge for chemists to define. Peeling away from the microscope, however, it is really simple. A solid is a structure with a definite shape and volume. That is, if you stick a round solid into a square box, it will still be round. It will also still be the same size. This definition implies a certain structural rigidity about solids, making them a fascinating field of study because of the numerous practical applications--like building bridges, or making a lightweight cell phone.
Some types of intermolecular forces that could be going on in a solid include ionic bonding, covalent network bonding, and metallic bonding. Dipole-dipole interactions, hydrogen bonding, and dispersion forces are generally not strong enough to keep particles together in solid form. Ice is a special case where hydrogen bonding will hold it in a solid, and this only occurs because of the low temperature (which means molecules aren’t moving around much, so they don’t have the energy to escape hydrogen bonds). Sometimes, dispersion forces can be very strong if the molecule is just the right shape and size. This is what happens in fats. See the sub-lesson on intermolecular attraction for more information.
This is a great video if you’d like to learn more about solids:
Some types of intermolecular forces that could be going on in a solid include ionic bonding, covalent network bonding, and metallic bonding. Dipole-dipole interactions, hydrogen bonding, and dispersion forces are generally not strong enough to keep particles together in solid form. Ice is a special case where hydrogen bonding will hold it in a solid, and this only occurs because of the low temperature (which means molecules aren’t moving around much, so they don’t have the energy to escape hydrogen bonds). Sometimes, dispersion forces can be very strong if the molecule is just the right shape and size. This is what happens in fats. See the sub-lesson on intermolecular attraction for more information.
This is a great video if you’d like to learn more about solids:
Liquids
In liquid form, molecules are loosely bonded together; while liquids have definite volume, they do not have definite shape. For example, when you pour water from a beaker to a tub, the amount of water you pour is the same, but the shape is different. This is because molecules can move (relatively) freely in a liquid, whereas in a solid they're pretty much stuck in one place.
Note that you can pour a liquid. This is because the attraction between molecules is strong enough to hold the particles together (unlike in a gas), but not quite so strong that they are fixed in one spot (as they are in a solid). So, when you pour a liquid, the bonds are just sliding past each other, allowing the molecules to cascade down like a fountain. It's pretty cool.
Note that you can pour a liquid. This is because the attraction between molecules is strong enough to hold the particles together (unlike in a gas), but not quite so strong that they are fixed in one spot (as they are in a solid). So, when you pour a liquid, the bonds are just sliding past each other, allowing the molecules to cascade down like a fountain. It's pretty cool.
Common types of intermolecular forces between the molecules in a liquid include dipole-dipole interactions and hydrogen bonding. These are strong, but not so strong that they aren’t flexible. Dispersion forces can hold a liquid together if the molecules are pretty long and can pack together pretty well (if they pack together really well they could even make solids). This is what happens in oils. See the sub-lesson on intermolecular attraction for more information.
The stronger the intermolecular attraction between particles in a liquid, the more viscous, or thick, it will be (think about pouring molasses as compared to water). The density of the liquid also influences the viscosity, but you'll learn more about this later.
Solids can become liquids by adding heat, like in melting. Since heat is adding energy, this causes the molecules to move faster, which allows them to escape their bonds and get away from each other.
This is a great video on liquids, if you’d like to know more:
The stronger the intermolecular attraction between particles in a liquid, the more viscous, or thick, it will be (think about pouring molasses as compared to water). The density of the liquid also influences the viscosity, but you'll learn more about this later.
Solids can become liquids by adding heat, like in melting. Since heat is adding energy, this causes the molecules to move faster, which allows them to escape their bonds and get away from each other.
This is a great video on liquids, if you’d like to know more:
(Mandatory disclaimer: Please do not put gallium in your mouth. I mean, it’s not particularly toxic, but, like, why?)
Gases
If solids are held together by strong bonds and liquids weak ones, gases, then, contain molecules that do not bond with one another. As a result, gas particles are free to float around wherever they want, taking up as much space as they want. They do not have a definite volume or shape. In other words, they will spread out (expand, or increase in volume) or condense to fill the container they are in, and they'll take on it's shape, too. Gas molecules are also very far apart from each other. To understand this, try grabbing a fistful of air--rather hard, isn't it? (Air is mostly empty space). So, basically, gases are just loners who do whatever they want—or at least try.
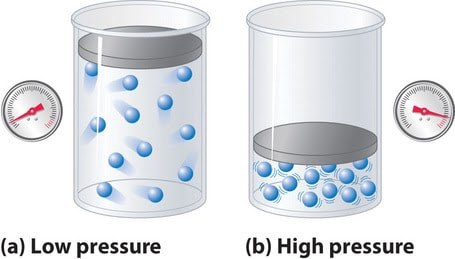
When there are lots of gas molecules in a small space, it is said to have a high pressure. If the container isn't strong enough to withstand this pressure, it might burst! (Think of an overfull balloon). If we decrease the volume of a rigid enough container, the gas just gets squished together and ends up very high pressure. So, maybe gases don’t so much do what they want as what their container wants. That somehow seems less cool.
Since gases don't have a definite volume, they can be easily compressed (pushed into a smaller volume). This causes an increase in pressure.
Likewise, gases take up more space when we remove pressure, as demonstrated in this video:
Likewise, gases take up more space when we remove pressure, as demonstrated in this video:
If any intermolecular attraction exists between gases, it is very weak (for example, weak dispersion forces).
Liquids can become gases when significant heat is added, like when we boil water. This happens for the same reason as the transition from solid to liquid: added kinetic energy means molecules can escape their bonds. Liquids can also become gases when significant pressure is removed, like in this video of boiling water in a vacuum:
Liquids can become gases when significant heat is added, like when we boil water. This happens for the same reason as the transition from solid to liquid: added kinetic energy means molecules can escape their bonds. Liquids can also become gases when significant pressure is removed, like in this video of boiling water in a vacuum:
This is a great video on the properties of gases:
We’ll talk more about gases later.
Plasma
Plasma is cool, and not very common. You may have heard of plasma in some other context. Plasma T.V.’s, neon signs, etc. But what exactly is plasma?
Well, plasma is an ionized gas. By heating up the gas, atoms/molecules gain energy. This causes gas to form intermolecular bonds (because of the ions participating in electrostatic interactions, like two ends of a magnet), creating plasma.
Plasma is similar to gases in that both have neither a definite shape nor a definite volume.
Well, plasma is an ionized gas. By heating up the gas, atoms/molecules gain energy. This causes gas to form intermolecular bonds (because of the ions participating in electrostatic interactions, like two ends of a magnet), creating plasma.
Plasma is similar to gases in that both have neither a definite shape nor a definite volume.
This is a nice video on plasma:
Summary
You should understand:
- That molecules are closest together and bound by the strongest intermolecular forces in solids. Solids have definite shape and volume.
- That molecules are medium-close together and bound by medium-strong intermolecular forces in liquids. Liquids have definite volume, but not shape.
- That molecules are far apart and bound by weak or no intermolecular forces in gases. Gases have neither definite volume nor shape.
Learning Activity
Content contributors: Rebecca Deng, Emma Moulton