Gas Laws
How Do Gases Behave?
Gases are a great model for starting off our understanding of how matter actually behaves when we change things like heat and pressure, because they change the most under these conditions. Other states of matter follow similar general trends, but we won't worry too much about them for now.
There is a set of rules that chemists have decided describe gases really well. They’re not perfect, but they are super useful, and good enough. These are called the gas laws. We won’t go into a ton of detail in this lesson, but there are a few relationships that you should understand just for the sake of being a well-educated person (and not blowing stuff up on accident). We could get more precise with these, and you could calculate stuff pretty accurately (so you would be able to know, for example, exactly how much you could heat up a container before it exploded), but we’ll focus on the basics here and save the calculations for later in your chemistry career.
There are four properties that we look at when we look at gas laws. They are pressure, temperature, volume, and the amount (number of molecules) of gas. Here, we will look at how these factors influence each other.
There is a set of rules that chemists have decided describe gases really well. They’re not perfect, but they are super useful, and good enough. These are called the gas laws. We won’t go into a ton of detail in this lesson, but there are a few relationships that you should understand just for the sake of being a well-educated person (and not blowing stuff up on accident). We could get more precise with these, and you could calculate stuff pretty accurately (so you would be able to know, for example, exactly how much you could heat up a container before it exploded), but we’ll focus on the basics here and save the calculations for later in your chemistry career.
There are four properties that we look at when we look at gas laws. They are pressure, temperature, volume, and the amount (number of molecules) of gas. Here, we will look at how these factors influence each other.
How Does the Amount of Gas Change Volume?
We’ll start with the relationship between the amount of gas and the volume, because this one is the most intuitive. Think, what will happen to the volume (size) of, say, a balloon or a car tire if you add more air to it?
If you said that it gets bigger (larger volume), then you’re exactly right. If not, go blow up a balloon and prove it to yourself. The more air you add (the more you blow into it), the bigger it gets.
If you said that it gets bigger (larger volume), then you’re exactly right. If not, go blow up a balloon and prove it to yourself. The more air you add (the more you blow into it), the bigger it gets.
How Does the Amount of Gas Change Pressure?
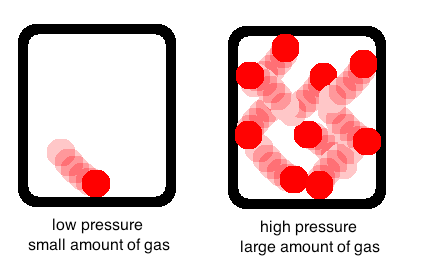
Now lets move on to a somewhat more complicated relationship (it’s still not too bad): pressure and the amount of gas. For this example (and all the examples we will do in this class), we will assume that these are the only two variables changing. In other words, you have a completely rigid container, so the volume won’t change even if the pressure does. In the real world, this isn’t always the case, but go with it.
In order to understand this relationship, we must first understand what pressure is. Pressure is the amount that a gas pushes out on its container. Think of gas molecules as a bunch of little balls bouncing around in their container. This actually describes what happens pretty well. Now, the little balls are eventually going to bump into each other, and they will bump into the walls of the container. The more they bump into each other—and, more importantly, the more they bump into the walls—the higher the pressure will be.
So, let’s go back to the relationship between the amount of gas and the pressure. Think about our container of bouncing balls again. If we only have one ball, it is not going to collide with the walls (or other balls, since there are none) very much. If we have ten balls, on the other hand, they’re going to get pretty crowded pretty fast and bump into the walls all the time. So, what is the relationship between the amount of gas (the number of balls) and the pressure (the amount the balls hit the walls)? Remember, we are assuming the container stays the same size.
Pressure goes up as the amount of gas goes up! This is why a balloon will eventually burst if you keep blowing air into it—eventually, the container (the balloon) isn’t really stretchy anymore, so the pressure goes up as you keep adding air. When pressure goes up too much, the balloon will burst (the same thing will happen with any container). If you got this, give yourself a pat on the back and move on with the lesson. If not, take a moment to reread and digest what I have described. Try drawing it out for yourself.
In order to understand this relationship, we must first understand what pressure is. Pressure is the amount that a gas pushes out on its container. Think of gas molecules as a bunch of little balls bouncing around in their container. This actually describes what happens pretty well. Now, the little balls are eventually going to bump into each other, and they will bump into the walls of the container. The more they bump into each other—and, more importantly, the more they bump into the walls—the higher the pressure will be.
So, let’s go back to the relationship between the amount of gas and the pressure. Think about our container of bouncing balls again. If we only have one ball, it is not going to collide with the walls (or other balls, since there are none) very much. If we have ten balls, on the other hand, they’re going to get pretty crowded pretty fast and bump into the walls all the time. So, what is the relationship between the amount of gas (the number of balls) and the pressure (the amount the balls hit the walls)? Remember, we are assuming the container stays the same size.
Pressure goes up as the amount of gas goes up! This is why a balloon will eventually burst if you keep blowing air into it—eventually, the container (the balloon) isn’t really stretchy anymore, so the pressure goes up as you keep adding air. When pressure goes up too much, the balloon will burst (the same thing will happen with any container). If you got this, give yourself a pat on the back and move on with the lesson. If not, take a moment to reread and digest what I have described. Try drawing it out for yourself.
How Does Temperature Change Pressure?
Now lets consider a third (and our last) relationship. There are others that we could talk about, but these are the ones you’ll see most often in your everyday life, so these are the only ones we’ll discuss. What is the relationship between temperature and pressure?
Remember from the last few sublessons that temperature is the amount of kinetic energy something has. In other words, it is how fast the balls are bouncing around our container. Since we now know that pressure is how much these balls bump into the sides of the container, it should make sense that as temperature goes up, pressure goes up. If balls are bouncing faster, they’re going to bump into the walls more often. For a cool example of this, watch this video:
Remember from the last few sublessons that temperature is the amount of kinetic energy something has. In other words, it is how fast the balls are bouncing around our container. Since we now know that pressure is how much these balls bump into the sides of the container, it should make sense that as temperature goes up, pressure goes up. If balls are bouncing faster, they’re going to bump into the walls more often. For a cool example of this, watch this video:
Whoa, what happened there? Well, aerosol cans contain gas under pressure. When we heat these cans up (say, by throwing them into a fire) the pressure gets even higher and they explode. Don’t try this at home, kids.
For another experiment that you can try at home (with the permission and supervision of your relevant responsible adult), check out this video:
Summary
This video provides a good summary of the relationships we discussed in this lesson:
This video shows you a semi-rigid container, where we see some of the effects of a rigid container (increase in pressure as we add more molecules or increase temperature) and some of the effects of a flexible container (increase in volume as we add more molecules or increase temperature). We are most interested in you understanding what happens in “perfect” systems that are either perfectly rigid or perfectly flexible—either only pressure changes or only temperature changes—but it is very helpful to understand that the real world usually has something in between.
If you’d like a deeper dive into the gas laws, with just a scosche of nerdy history, this video is great:
You should understand:
- As we add more gas to a container, the volume will go up (if the container is stretchy like a balloon).
- As we add more gas to a container, the pressure will go up (if the container is not stretchy, like a glass jar; if it were, volume would increase).
- As we increase the temperature of a container, the pressure will go up (if the container is not stretchy—if it were, volume would increase).
Learning Activity
Content contributors: Emma Moulton