The Periodic Table
Introduction
The Periodic Table of the Elements (which we just call the Periodic Table, for short) is basically the holy grail of science—only instead of dreaming to one day discover it, it is conveniently printed in the back of virtually all chemistry textbooks. (Check under the resources tab if you haven't seen ours yet!)
This video gives a fantastic overview of the Periodic Table:
This video gives a fantastic overview of the Periodic Table:
So, the Periodic Table is pretty cool and super useful. But, what makes the Periodic Table so cool and useful, you ask? Why do scientists love it so much? The answer is quite simple—we love it because it makes our lives easier. By conveniently organizing every element known to man into periods and groups based on their distinct properties, we can predict exactly how an element will behave without actually knowing anything about that particular element. Though you may not be able to fully understand this point now, it will become increasingly apparent as you journey on in your chemistry career, periodic table in tow.
Here are the most important things you should understand about the Periodic Table, which will make it a useful tool to you as you continue in your Chemistry learning:
Here are the most important things you should understand about the Periodic Table, which will make it a useful tool to you as you continue in your Chemistry learning:
5 Things You Should Know About the Periodic Table
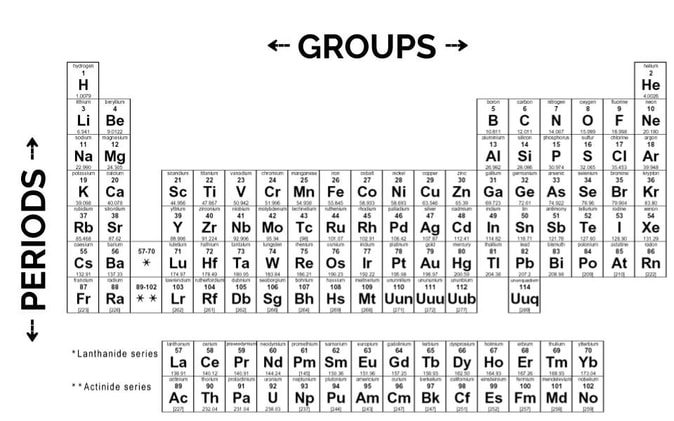
1. The Periodic Table is Divided into periods and groups. Periods are the rows. Elements in the same period share the same maximum energy level. Groups are the columns. Elements in the same group share similar reactivity patterns, because they share the same number of valence electrons, which are the electrons in the outermost shell or "rung" of the atom. Because they are closest to the outside and highest in energy, they are most readily available to interact with other atoms.
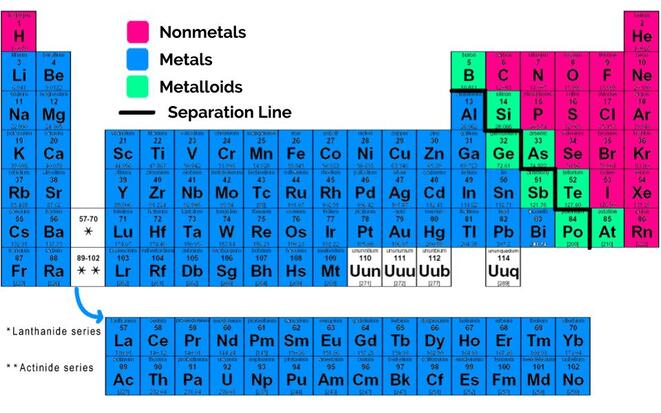
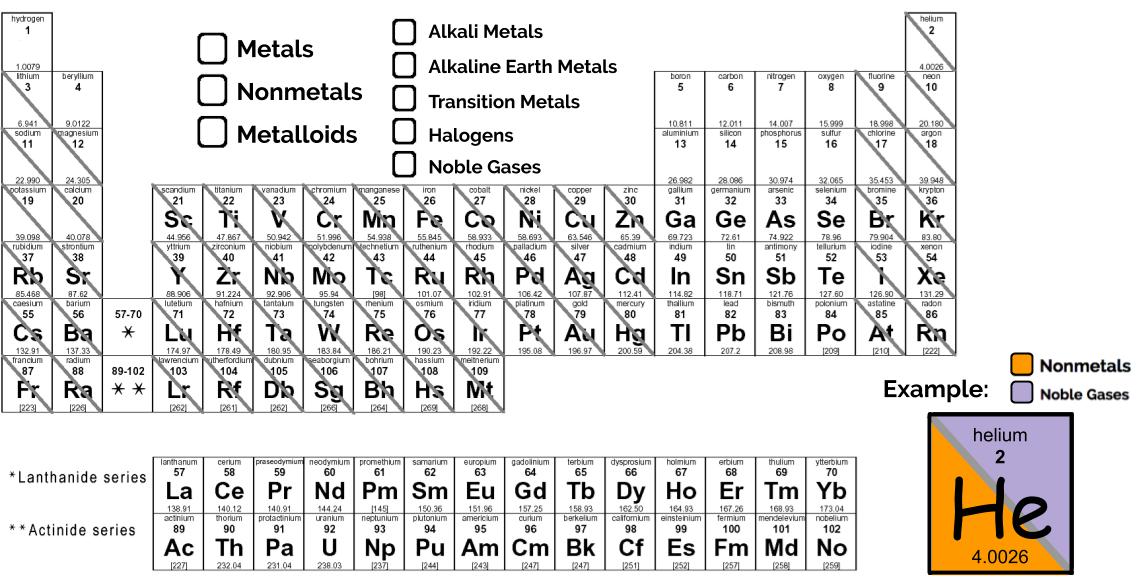
2. Elements on the Periodic Table are divided into metals, nonmetals, and metalloids. Metals tend to be malleable, shiny, and ductile. They fall to the left of the zig-zaggy separation line seen in the picture. Nonmetals tend to be either gases or brittle solids. Unlike metals, they are poor conductors of heat and electricity. They fall to the right of the zig-zag line. Metalloids consist of all the elements that touch the zig-zag line (except aluminum!). They have some properties of metals and some properties of nonmetals.
3. Elements are organized by atomic number, which tend to correspond to mass number. An element's atomic number is the number of protons that it has. Unless something is an ion, meaning it has extra or fewer electrons than usual, the number of electrons is the same as the number of protons, which is the same as the atomic number. The atomic number gets bigger as you go across a period—hydrogen's is 1, helium's is 2, lithium's is 3, and so on. The atomic mass is the weight of the element in atomic mass units (amu). It is about equal to the number of protons plus the number of neutrons, and tends to get bigger with atomic number.
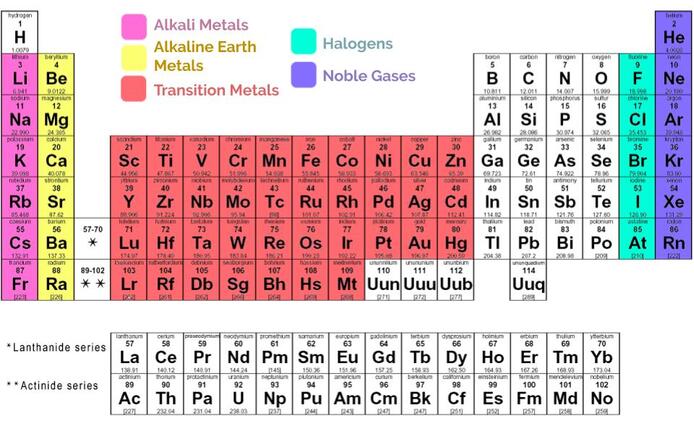
4. There are several families of elements, which all have similar properties. They are:
- Alkali metals: Lithium, sodium, potassium, etc. These metals are highly reactive—you'd have a hard time even finding them by themselves in nature. They like to bind to the halogens.
- Alkaline earth metals: Beryllium, magnesium, calcium, etc. Also very highly reactive, but not quite as much as the Alkali metals.
- Transition Metals: What you typically think of as metals. Include gold, silver, platinum, and chrome (chromium). Strong, malleable, and make great conductors.
- Halogens: The next to last column on the periodic table. Include fluorine, chlorine, and iodine. Very reactive, especially with alkali and alkaline earth metals. They are generally found in nature as diatomic gases.
- Noble gases: The "royalty" of the periodic table. Think they’re too good to react with anything else, so they stick all by their lonesomes and don’t bother with any of those other low class elements. If you prefer more scientific terms, they are highly stable because they have a full valence shells (every electron in the valence shell already has enough friends, so they don't need to go hang out with other atoms), so they are unreactive.
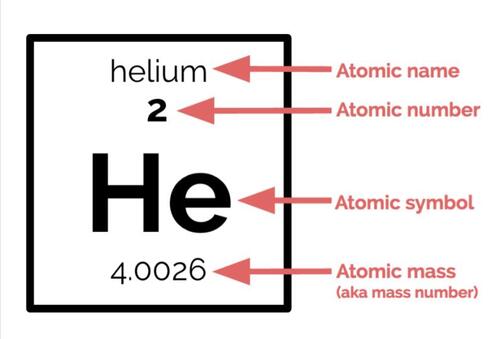
5. Each element is given a 1- to 2-letter symbol. Some of them are logical—hydrogen is H, helium is He, oxygen is O, and so forth. Some of them do not make as much sense—for example, the symbol for sodium (Na) is based off of the Latin word natrium rather than the English word. Don't feel like you need to memorize all of them, but it will be very useful in your chemistry career if you know the symbols for at least the first 20 elements. (You do not have to memorize them for this class).
Summary
You should understand:
You will have more practice with using the Periodic Table throughout this unit, so don't worry if you aren't quite yet perfect. But, the sooner you figure it out, the easier the rest of Chemistry will become!
- The relationship between and element's location on the periodic table, its group, the number of valence electrons that it has, and its reactivity patterns.
- The differences between metals, nonmetals, and metalloids, and where each of these are found on the periodic table.
- The differences between alkali metals, alkaline earth metals, transition metals, halogens, and noble gases—especially in terms of their reactivity—and where each of these are found on the periodic table.
- Where to find an element's atomic name, atomic symbol, atomic number, and atomic mass, and how atomic number and atomic mass relate to the number of protons, neutrons, and electrons.
You will have more practice with using the Periodic Table throughout this unit, so don't worry if you aren't quite yet perfect. But, the sooner you figure it out, the easier the rest of Chemistry will become!
Learning Activity
Now is your chance to get acquainted with your new best friend, the Periodic Table! (And those first 20 elements!) Either print off the following periodic table and color it in by hand or upload it to a computer-based illustrator program like Paint.
Each element should be identified as either a metal, a nonmetal, or a metalloid by coloring in the box (or half of the box, if there's a diagonal line running through it). You can choose whatever color scheme you want, just be sure to fill in the legend with the same color that you use to identify each element. Be sure to choose distinct colors for each classification so it's easy to tell what's what.
Each box with a diagonal line running through it should be marked as both a metal/nonmetal/metalloid AND according to its family, as illustrated in the example. Those with no diagonal line need only be marked as a metal/nonmetal/metalloid.
Also fill in the chemical symbol of the first 20 elements. Since you don't have to memorize them, you can just look them up online or using the Periodic Table included in our Resources section. This will help you to get more familiar with the elements we use most often.
Have fun!
Tip: Right-click the image to see the drop down box, which (depending on what browser you use) should give you the option to "Download Image," "Save Image As," or "Print".
Each element should be identified as either a metal, a nonmetal, or a metalloid by coloring in the box (or half of the box, if there's a diagonal line running through it). You can choose whatever color scheme you want, just be sure to fill in the legend with the same color that you use to identify each element. Be sure to choose distinct colors for each classification so it's easy to tell what's what.
Each box with a diagonal line running through it should be marked as both a metal/nonmetal/metalloid AND according to its family, as illustrated in the example. Those with no diagonal line need only be marked as a metal/nonmetal/metalloid.
Also fill in the chemical symbol of the first 20 elements. Since you don't have to memorize them, you can just look them up online or using the Periodic Table included in our Resources section. This will help you to get more familiar with the elements we use most often.
Have fun!
Tip: Right-click the image to see the drop down box, which (depending on what browser you use) should give you the option to "Download Image," "Save Image As," or "Print".
Content contributors; Emma Moulton and Emily Zhang