Intermolecular Attraction
Molecules Attracting Molecules
So far, we have been talking about intramolecular attraction, or attraction within a molecule. But, obviously, molecules don't tend to come in a party of one. When you look at a chair, you aren't looking at a molecule, you're looking at a chair. So, then, we need to talk about another type of interaction if we want to really understand the universe. This is called intermolecular attraction and is what holds a group of molecules together.
There are several types of intermolecular bonds that can form. Some are pretty strong. Some are pretty fluid, more like molecules dancing around each other, coming into attraction for a brief moment, and then flitting away to dance with other molecules. It’s poetic. Like a song. A song that sounds like this one:
There are several types of intermolecular bonds that can form. Some are pretty strong. Some are pretty fluid, more like molecules dancing around each other, coming into attraction for a brief moment, and then flitting away to dance with other molecules. It’s poetic. Like a song. A song that sounds like this one:
The relative strength of these intermolecular attractions determines many of the physical and chemical properties of elements and molecules, including some pretty fundamental ones, like whether they are solids, liquids, or gases. So let’s take a deeper dive into what these different types of intermolecular attractions are.
Ionic Bonds
Ionic bonds are a kind of weird mashup between an intramolecular bond and an intermolecular bond, so I'll include it here, but it's really nothing new. They are very strong, because they are the result of electrostatic interactions of complete, permanent charges: That is, completely and forever giving up or receiving electrons. This is in contrast to the partial or temporary charges that we’ll learn about in some of the other intermolecular bond types. Because these bonds are so strong, they tend to result in solid compounds.
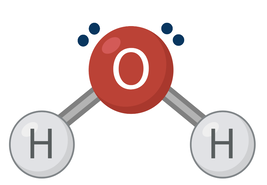
Dipole-Dipole Interactions. Take a look at this picture of a water molecule:
Dipole-Dipole Interactions. Take a look at this picture of a water molecule:
Water is polar. This means that it has a negative end and a positive end. The simplest way to recognize this is by, going back to the picture, seeing that one end of the molecule has free electrons (those dots). As you know, electrons are negative. So, the end of water with free electrons must also be negative. The other end of the molecule is bonded to hydrogen. Hydrogen has protons, and protons are positive. So, that end of water must be positive (at least compared to the other end).
The other, more important reason that water is polar is because oxygen is more electronegative than hydrogen. As you’ve learned, electronegativity means that oxygen is a greedy electron hog. It will pull the electrons away from hydrogen and towards itself. And, again, since electrons are negative, this makes the oxygen atom slightly more negative than the hydrogen atoms. This creates a partial charge or dipole.
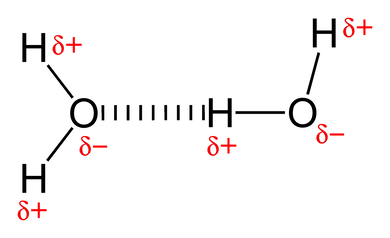
Since water has a negative end and a positive end, and opposites attract, the positive end of one water molecule is attracted to the negative end of another water molecule, and the two molecules stick together! Amazing!
The other, more important reason that water is polar is because oxygen is more electronegative than hydrogen. As you’ve learned, electronegativity means that oxygen is a greedy electron hog. It will pull the electrons away from hydrogen and towards itself. And, again, since electrons are negative, this makes the oxygen atom slightly more negative than the hydrogen atoms. This creates a partial charge or dipole.
Since water has a negative end and a positive end, and opposites attract, the positive end of one water molecule is attracted to the negative end of another water molecule, and the two molecules stick together! Amazing!
When a molecule becomes charged because of electronegativity, it is called a dipole moment (see the word pole, as in polar, in there?). As such, the interaction in which the negative end of a polar molecule sticks to the positive end of another—like with water molecules—is called a dipole-dipole interaction. It can occur between any polar molecules, not just water. In fact, the attraction in water is actually a special kind of dipole-dipole interaction called hydrogen bonding. You'll hear this phrase a lot later on because it is important to life.
Most substances that have this interaction are usually gasses or liquids at room temperature.
Note: this type of interaction is somewhat similar to an ionic bond in that charges are interacting to hold stuff together. But, it is important to differentiate between the two. In an ionic bond, the whole molecule is charged, and it interacts with another molecule with a whole (opposite) charge. In this case only part of the molecule is charged, so it can interact with other molecules of the same type.
Hydrogen Bonding. As mentioned, hydrogen bonding is a special case of dipole-dipole interaction. However, hydrogen bonds are much stronger than dipole-dipole because the hydrogen atom is virtually left “naked,” because it doesn’t have electrons “protecting” it—it doesn’t have unshared electrons, because hydrogen doesn’t have or need an octet. This makes it extra positive, compared to things like carbon, which can participate in dipole-dipole interactions, but not hydrogen bonding.
As you may have guessed from the name, hydrogen bonding always involves hydrogen. Its negative counterpart will always be a very electronegative atom, like N, O, or F (reminder: the closer you are to fluorine on the periodic table, the more electronegative you are), because these are the biggest electron hogs.
London Dispersion Forces. London dispersion forces are very weak and occur between neutral (uncharged), nonpolar molecules. They, like the other types of intermolecular attraction, rely on positive and negative charges interacting. However, unlike the other types of intermolecular attraction, these charges are not permanent (hence why London dispersion forces are weak). To understand this, we must take a step back to the structure of the atom.
Electrons are not stuck in place. This is one of the fall-backs of drawing models on paper-- we can't see the motion. But, in reality, motion does occur, and, in fact, it occurs quite a lot, and quite fast. Electrons are always moving. ALWAYS.
What does this mean in terms of intermolecular attraction? It means that electrons, just by chance, can end up on one side of a molecule. They won't stay there forever, but for this fleeting moment, the molecule is polar― one side is negative, leaving the other positive and stripped of electrons. So, in this fleeting moment, it can stick to another molecule, which also happens to have a dipole at this moment in time.
This video shows London dispersion forces pretty well:
Most substances that have this interaction are usually gasses or liquids at room temperature.
Note: this type of interaction is somewhat similar to an ionic bond in that charges are interacting to hold stuff together. But, it is important to differentiate between the two. In an ionic bond, the whole molecule is charged, and it interacts with another molecule with a whole (opposite) charge. In this case only part of the molecule is charged, so it can interact with other molecules of the same type.
Hydrogen Bonding. As mentioned, hydrogen bonding is a special case of dipole-dipole interaction. However, hydrogen bonds are much stronger than dipole-dipole because the hydrogen atom is virtually left “naked,” because it doesn’t have electrons “protecting” it—it doesn’t have unshared electrons, because hydrogen doesn’t have or need an octet. This makes it extra positive, compared to things like carbon, which can participate in dipole-dipole interactions, but not hydrogen bonding.
As you may have guessed from the name, hydrogen bonding always involves hydrogen. Its negative counterpart will always be a very electronegative atom, like N, O, or F (reminder: the closer you are to fluorine on the periodic table, the more electronegative you are), because these are the biggest electron hogs.
London Dispersion Forces. London dispersion forces are very weak and occur between neutral (uncharged), nonpolar molecules. They, like the other types of intermolecular attraction, rely on positive and negative charges interacting. However, unlike the other types of intermolecular attraction, these charges are not permanent (hence why London dispersion forces are weak). To understand this, we must take a step back to the structure of the atom.
Electrons are not stuck in place. This is one of the fall-backs of drawing models on paper-- we can't see the motion. But, in reality, motion does occur, and, in fact, it occurs quite a lot, and quite fast. Electrons are always moving. ALWAYS.
What does this mean in terms of intermolecular attraction? It means that electrons, just by chance, can end up on one side of a molecule. They won't stay there forever, but for this fleeting moment, the molecule is polar― one side is negative, leaving the other positive and stripped of electrons. So, in this fleeting moment, it can stick to another molecule, which also happens to have a dipole at this moment in time.
This video shows London dispersion forces pretty well:
The odds that all the electrons in a molecule would just happen to end up on one side of it may seem pretty small and insignificant. Really, what are the chances that, say, 40 electrons in a molecule end up on the same side of the molecule at the same time, and in close proximity to another molecule which also just happens to have 40 electrons on the same side of the molecule? Fat chance, right? This is just a guess, but the odds are probably greater that you will win the lottery, buy an NFL team, and watch them win the Super Bowl 5 years in a row. (Okay, maybe it's not that rare, but you get the idea).
So, how can this possibly be a significant source of intermolecular attraction? Well, for one thing, not all of the electrons have to be on the same side of the molecule for London dispersion forces to work. Just a majority of them. This is a lot more likely. For another thing, there are billions upon trillions of molecules in even a relatively small volume of a substance. (For example, if molecules were the size of cupcakes, 18 mL of water would fill approximately 12 billion trillion Olympic-sized swimming pools). This means that even though it might be rare for any two given molecules to be held together by London dispersion forces, they’re actually not that uncommon if you look at the entire volume of substance. Alone they are weak, but together they are mighty. In fact, it's what allows a gecko to climb up glass:
These forces are most often found among gasses, and they’re sometimes found in liquids.
Summary
You should understand:
- The relative strengths of ionic bonds, hydrogen bonding, dipole-dipole interactions, and London dispersion forces, and how this relates to whether a charge/dipole is permanent, temporary, complete, or partial.
- Which forces are most commonly found in solids, liquids, and gasses, and how that relates to the strength of the interaction. We’ll also talk about this more specifically later on.
Learning Activity
Content contributors: Rebecca Deng, Emma Moulton, Eli Levine, Emily Zhang