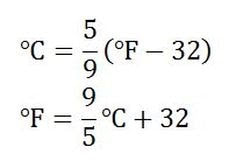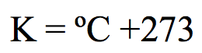Temperature
So far, you have learned about atoms, molecules, and some of the properties of these atoms and molecules. In other words, you have learned about matter. Stuff. Thermodynamics is a little different in that it focuses on the energy and motion associated with matter. We’ve given you some sneak peaks at thermodynamics, but we’ll talk about it a lot more specifically now.
Energy can be a little harder to think about sometimes because, unlike making a molecular model or looking at the states of matter, you can't see energy. It's just something that's just sort of… there. But, it is an extremely important topic in chemistry (and physics, and biology). Why? Because atoms and molecules don't just sit around in their Bohr models or Lewis dot structures all day, looking pretty. They do stuff. And when we start to understand thermodynamics, we can start to understand why and how they do stuff.
Energy can be a little harder to think about sometimes because, unlike making a molecular model or looking at the states of matter, you can't see energy. It's just something that's just sort of… there. But, it is an extremely important topic in chemistry (and physics, and biology). Why? Because atoms and molecules don't just sit around in their Bohr models or Lewis dot structures all day, looking pretty. They do stuff. And when we start to understand thermodynamics, we can start to understand why and how they do stuff.
Temperature
You probably have an intrinsic idea of what temperature is. That is, you kind of have a feel for what it is even if you couldn't put together the exact definition. It is a measure of how hot or cold something is. 91° F is hotter than 27°F. You could go swimming if it were 91° F outside. You would probably prefer not to if it were 27° F outside. But, what exactly is temperature? How is it measured? And why do we even talk about it?
Temperature, Energy, and Motion
Temperature is a measure of the average amount of kinetic energy a group of molecules has—that is, the amount of energy the molecules have because they are moving around. If molecules are buzzing back and forth really fast, constantly bumping into each other and other stuff, they have a lot of kinetic energy. Based on the definition of temperature (high temperature is high kinetic energy), they therefore have a higher temperature, and they will feel warm. This is because one of the ways energy manifests itself is as heat. You'll learn more about this later on. On the contrary, if molecules are just moseying about, taking their sweet time as they go, we say that they are low in energy. This also means that they will have a low temperature and feel cold.
Now, not all of the molecules in a group will have the same energy—some might be moving really fast while others are moving really slow, and the rest might be somewhere in between. This is why we call temperature the average kinetic energy of particles—so if most of the particles are moving fast, and some are moving slow, on average you will have a higher temperature.
Now, not all of the molecules in a group will have the same energy—some might be moving really fast while others are moving really slow, and the rest might be somewhere in between. This is why we call temperature the average kinetic energy of particles—so if most of the particles are moving fast, and some are moving slow, on average you will have a higher temperature.
Molecules of different speeds moving around. This sample probably has a pretty high temperature.
This video gives a little more detail on temperature, heat, and kinetic energy if you’re looking for some more clarification:
Temperature Scales
Now, the really handy thing about temperature scales is that they allow us to quantify the amount of energy particles have. We don't just say "Oh, these guys are moving a little faster than those guys, and so they must have a little more energy," we say, "Oh, these guys have a temperature of 60° F and those guys have a temperature of 55° F." The rest is implied with the numbers. It's just a lot easier to talk about science if we can be precise with things—and numbers allow us to do this.
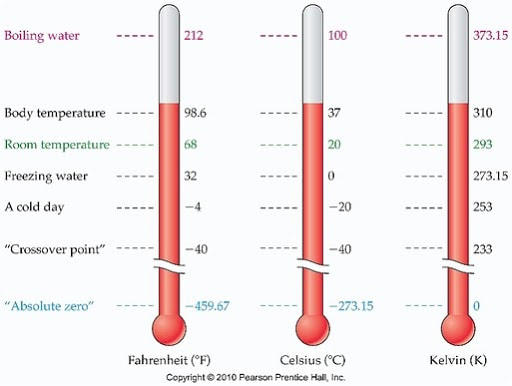
There are three commonly used temperature scales. You have probably heard of Fahrenheit and Celsius. Celsius is the most commonly used worldwide, and it is generally the scale we use in science. Fahrenheit is really only used in the US. The third scale is called Kelvin, and it is the most "scientific" of the three—we mostly use it in making calculations and such, but occasionally we report temperatures in Kelvin.
Here is a chart that compares the three:
There are three commonly used temperature scales. You have probably heard of Fahrenheit and Celsius. Celsius is the most commonly used worldwide, and it is generally the scale we use in science. Fahrenheit is really only used in the US. The third scale is called Kelvin, and it is the most "scientific" of the three—we mostly use it in making calculations and such, but occasionally we report temperatures in Kelvin.
Here is a chart that compares the three:
Click to enlarge. Note that we do not say "degrees" Kelvin. We just say Kelvin. This is because Kelvin is an absolute scale. It's just like how you wouldn't say "degrees" inches or "degrees" seconds.
As you can see in the chart, Fahrenheit is weird. It doesn't really make much sense why the numbers are picked the way that they are. (Okay, there is some logic to it if you really dig, but it's definitely not obvious logic).
Celsius makes a lot of sense. Water freezes at zero degrees, and boils at a hundred degrees. These are nice round numbers, especially considering the importance of water. We will use this scale often in science, so it is handy to be able to convert between Fahrenheit and Celsius (because Fahrenheit is weird and we never really use it but if you live in the US it's probably the one you'll hear about):
Celsius makes a lot of sense. Water freezes at zero degrees, and boils at a hundred degrees. These are nice round numbers, especially considering the importance of water. We will use this scale often in science, so it is handy to be able to convert between Fahrenheit and Celsius (because Fahrenheit is weird and we never really use it but if you live in the US it's probably the one you'll hear about):
So, all you need to do to go from Fahrenheit to Celsius is subtract 32 and multiply the whole thing by 5/9. To go the other way, multiply the temperature in Celsius by 9/5 and add 32 to that. Alternatively, if you just need a rough estimate, you can try the trick in this video:
Kelvin (arguably) makes the most sense of the three scales. Why? Because zero isn't some arbitrary number, or some number that only makes sense if you're comparing it to something else—zero means zero, nothing. At this theoretical temperature, called absolute zero, all motion stops. (Which makes total sense, because motion is energy and energy is temperature). We use it in a lot of equations because it is impossible to have a negative number, which makes them work out nicely. So, then, you might find it useful to be able to convert from Celsius to Kelvin.
Summary
So, now I re-pose to you my original questions: See if you can answer them. What is temperature? How is it measured? Why do we even talk about it?
If you’re still having trouble (or if you just want a good recap), this video gives a great overview of some of the most important things we discussed in this lesson:
If you’re still having trouble (or if you just want a good recap), this video gives a great overview of some of the most important things we discussed in this lesson:
You should understand:
- How temperature relates to the kinetic energy of molecules.
- How to convert among Celsius, Kelvin, and Fahrenheit scales of temperature.
Learning Activity
Content contributors: Emma Moulton