Macromolecules for Doing Things: Proteins and Nucleic Acids
What Do Cells Do?
Cells do a lot of things that keep you alive. Mainly, they do chemical reactions that are catalyzed, or made faster (in this case, fast enough to be possible), by special proteins called enzymes. Basically, just about every useful thing that your cell does is made possible by a protein. They’re kind of a big deal.
Many basic cell processes involve making it possible for proteins to do stuff. These reactions also involve a lot of proteins doing stuff. For example, cell respiration, a chemical reaction catalyzed by enzymes (proteins) provides energy for proteins to do stuff. Another reaction, protein synthesis, a chemical reaction catalyzed by enzymes (including proteins), makes proteins based on instructions for DNA. Another reaction, DNA replication, which occurs before mitosis (cell replication), involves making new DNA so that all cells will have the instructions to make protein. And, you guessed it, DNA replication and mitosis are both chemical reactions that rely on enzymes, which are proteins. We’ll talk about a couple of these processes in a little more details in this class.
For a little more on the topic of what cells do, check out this quick overview of cell biology:
Many basic cell processes involve making it possible for proteins to do stuff. These reactions also involve a lot of proteins doing stuff. For example, cell respiration, a chemical reaction catalyzed by enzymes (proteins) provides energy for proteins to do stuff. Another reaction, protein synthesis, a chemical reaction catalyzed by enzymes (including proteins), makes proteins based on instructions for DNA. Another reaction, DNA replication, which occurs before mitosis (cell replication), involves making new DNA so that all cells will have the instructions to make protein. And, you guessed it, DNA replication and mitosis are both chemical reactions that rely on enzymes, which are proteins. We’ll talk about a couple of these processes in a little more details in this class.
For a little more on the topic of what cells do, check out this quick overview of cell biology:
How Do Cells Do Things?: Protein
So, proteins do stuff. Let’s get into more of the details of the types of things proteins do and what they’re made of.
Enzymes are the “Workhorse” of the Cell
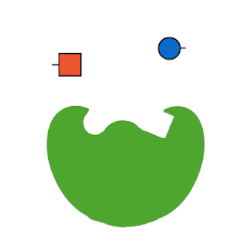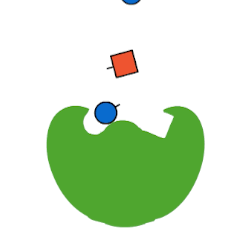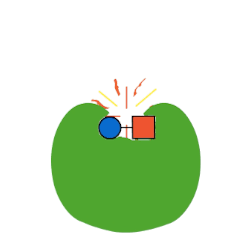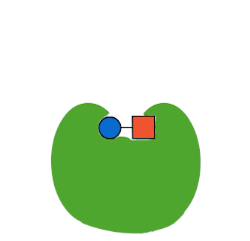
The first type of protein in living things is called an enzyme. Enzymes are often called “the workhorse of the cell” because they make a lot of the chemical reactions in our bodies possible. Now, technically, every reaction in our body could occur at some point without an enzyme. But, “at some point” in chemistry might be a really, really long time.
Life doesn’t have the luxury of waiting around for molecules to just randomly bump into each other and, even then, only maybe carry out a chemical reaction. Chemical reactions, especially the elaborate ones involved in life, take a lot of energy to make them happen. This energy is called activation energy. When molecules are just randomly floating around in solution, like in a test tube, they have to—just by chance—bump into each other at just the right angle, with enough kinetic energy to react, in a chemical environment that allows that reaction to occur (like high or low pH).
Enzymes are the “Workhorse” of the Cell
The first type of protein in living things is called an enzyme. Enzymes are often called “the workhorse of the cell” because they make a lot of the chemical reactions in our bodies possible. Now, technically, every reaction in our body could occur at some point without an enzyme. But, “at some point” in chemistry might be a really, really long time.
Life doesn’t have the luxury of waiting around for molecules to just randomly bump into each other and, even then, only maybe carry out a chemical reaction. Chemical reactions, especially the elaborate ones involved in life, take a lot of energy to make them happen. This energy is called activation energy. When molecules are just randomly floating around in solution, like in a test tube, they have to—just by chance—bump into each other at just the right angle, with enough kinetic energy to react, in a chemical environment that allows that reaction to occur (like high or low pH).
Enzymes fix all of these problems. Enzymes lower activation energy by taking a lot of the chance out of how molecules interact with each other. Enzymes bring the right molecules for a reaction together in just the right orientation, and provide just the right surrounding environment, to make it a lot easier—and so, so, so much faster—for biochemistry to occur. Thanks, enzymes!
This video provides a helpful overview of enzymes (don’t worry about inhibitors for this class, but it’s good for you to know that they exist):
Structural Proteins Provide Structure
The next type of protein in living things is called a structural protein. Much as the name implies, these proteins provide structure.
In humans, the most common structural protein is called collagen. Collagen basically forms a giant net that suspends all of your cells, keeping them in just the right place in your tissues and organs to carry out their jobs. Here’s a video of collagen at work:
The next type of protein in living things is called a structural protein. Much as the name implies, these proteins provide structure.
In humans, the most common structural protein is called collagen. Collagen basically forms a giant net that suspends all of your cells, keeping them in just the right place in your tissues and organs to carry out their jobs. Here’s a video of collagen at work:
Oh, oops, that was Spiderman. But, collagen works in basically the same way as Spiderman’s webshooters: It’s really strong, pretty stretchy, and is great at holding stuff together. Here’s an actual video of collagen:
Nope, Spiderman again, my bad....
Aced it.
Carrier Proteins Carry Stuff
The third type of protein in living things is the carrier protein. Like the name implies, these proteins carry other things, especially things that are very small or are hard to dissolve in water. One major example in animals (including humans) is hemoglobin, which carries oxygen through your blood and drops it off at the cells that need it most. Carrier proteins are also involved in transporting substances across the cell membrane, which we’ll learn more about when we talk about membrane transport.
Here’s a sneak peak:
Carrier Proteins Carry Stuff
The third type of protein in living things is the carrier protein. Like the name implies, these proteins carry other things, especially things that are very small or are hard to dissolve in water. One major example in animals (including humans) is hemoglobin, which carries oxygen through your blood and drops it off at the cells that need it most. Carrier proteins are also involved in transporting substances across the cell membrane, which we’ll learn more about when we talk about membrane transport.
Here’s a sneak peak:
Or, for more detail:
Signalling Proteins Are For Cellular Communication
The last major group of proteins is made up of signalling proteins. Signalling proteins are involved in signalling pathways. Signalling pathways are how cells in our body talk to each other to perform complex functions.
Basically, when one cell recognizes that something needs to be done (because of some other chemistry that happened to cause it to realize that), it releases a signalling molecule. That signalling molecule is then recognized by other cells, which somehow change their function to make that thing that needs to be done happen. This process typically involves a protein interacting with another protein, which then interacts with another protein, which then interacts with another protein, and so on, until the protein or proteins that do the thing that needs to be done are turned “on”.
The nuances of how this all works are a bit more complicated than we’ll go into in this class, but you should know that cells communicate with each other through biochemistry involving proteins. Here’s an example of a signalling pathway:
Talk about a game of telephone….
What Are Proteins Made Of?: Amino Acids
So, now we know that proteins either make chemical reactions happen, hold stuff together, move stuff, or tell cells to do stuff. So… basically, they do all the things. But, what are these über important structures made of? Well, as macromolecules, they are polymers. Their monomers are called amino acids.
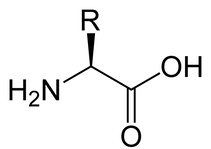
An amino acid consists of a “--COOH” group, an “amino” group, and an “R” group. The “R” isn’t actually a chemical structure, we just use it as a placeholder to describe the general structure.
An amino acid consists of a “--COOH” group, an “amino” group, and an “R” group. The “R” isn’t actually a chemical structure, we just use it as a placeholder to describe the general structure.
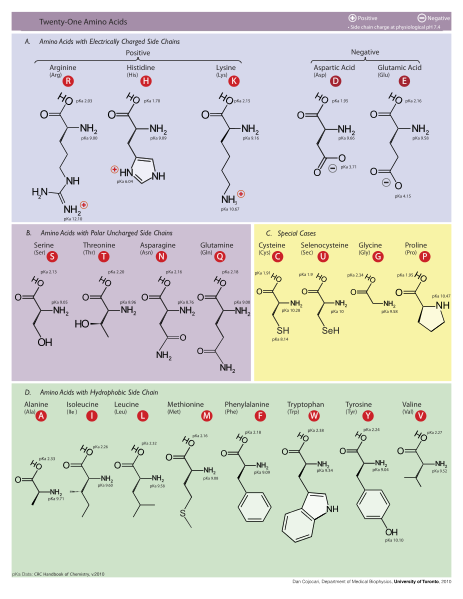
If you’ve been scratching your head, looking at your Periodic Table, trying to figure out what the heck kind of element is “R,” don’t! It’s not actually an element: we just use it as a placeholder to tell us that an amino acid could have any one of about 20 different sidechains, or functional groups. There are many different amino acids, all of which have a different chemical structure in place of the “R.” These different chemical structures and the different order that the amino acids are found in different proteins are what give each protein its unique structural and functional features. You don’t need to know the details of what the R groups look like or what kinds of things they do for this class, but you should know that they’re what make proteins unique and useful.
Amino acids have different sidechains, or R groups, that give the protein unique
structural and functional properties. Click to enlarge.
structural and functional properties. Click to enlarge.
How Are Proteins Made?: Nucleic Acids
At this point, you may be asking, “If proteins are so important, where do they come from?” The short answer is that they are made with the help of nucleic acids.
Of the different kind of macromolecules, I suspect you may not be as familiar with the term nucleic acid. So, what is a nucleic acid? I’ll give you a hint: DNA stands for DeoxyriboNucleic Acid.
DNA is a term that a lot of people know but not enough people really understand. You probably know that DNA makes you you, and you may also know it as your genes or your genome. It is found in every cell of your body (well, expect for your red blood cells…. They’re weird). It stores your genetic information. You get half of it from your mom and half from your dad. It is a permanent fixture of your cells and will never go away. And, fun fact, if you stretched out all the DNA in your body, it would stretch to the sun and back about 4 times. But what is DNA? What does it do?
Of the different kind of macromolecules, I suspect you may not be as familiar with the term nucleic acid. So, what is a nucleic acid? I’ll give you a hint: DNA stands for DeoxyriboNucleic Acid.
DNA is a term that a lot of people know but not enough people really understand. You probably know that DNA makes you you, and you may also know it as your genes or your genome. It is found in every cell of your body (well, expect for your red blood cells…. They’re weird). It stores your genetic information. You get half of it from your mom and half from your dad. It is a permanent fixture of your cells and will never go away. And, fun fact, if you stretched out all the DNA in your body, it would stretch to the sun and back about 4 times. But what is DNA? What does it do?
DNA is a double-helix: a popular icon of science and biology that you should
really understand as more than just a popular icon of science and biology.
really understand as more than just a popular icon of science and biology.
Well, through a process that we won’t get into in this class, DNA is transcribed into another, more temporary nucleic acid called RNA, or ribonucleic acid. This RNA is then translated into protein. You don’t have to know any of the details of this process for this class, but this video explains it, and it will probably help you to understand the purpose of DNA much better:
The short version of this video: DNA does not become protein, but it sure makes protein possible. Basically, DNA is the recipe for all of the proteins in your body. Since proteins are basically everything you are and do on a cellular level, that makes DNA the recipe for you! Thanks, DNA!
Summary
So, that’s biochemistry in a nutshell. Energy in the form of carbohydrates and fat, the stuff proteins do with that energy, and the DNA (and RNA) that form the recipe for those proteins. Just about everything you are and do distilled down to 4 major types of molecules. It’s absolutely amazing, and understanding the general purpose of these molecules will provide you with the background you need to understand what makes life possible.
Here’s a helpful recap of the four different types of macromolecules:
Here’s a helpful recap of the four different types of macromolecules:
You should understand:
- That proteins do most of the jobs of a cell:
- Enzymes: make the chemical reactions of life possible by putting substrates (the things reacting) in the right orientation to react with each other, lowering the activation energy of that reaction.
- Structural proteins like collagen provide shape and support to cells. They also bind cells together in tissues.
- Carrier proteins transport substrates in a very specific manner. One application of this is deciding what can go in and out of a cell.
- Signalling proteins allow cells to communicate with each other.
- Enzymes: make the chemical reactions of life possible by putting substrates (the things reacting) in the right orientation to react with each other, lowering the activation energy of that reaction.
- That proteins are made up of amino acids. There are many different types of amino acids, each of which gives the protein unique structural and functional abilities.
- That protein is made using the genetic instructions from the nucleic acid DNA, with the help of a nucleic acid called RNA.
Learning Activity
Contributors: Allan Wu and Emma Moulton