Composition of the Atmosphere
The atmosphere. You may know her as the air we breathe. She’s responsible for the wind, the rain, life, and all sorts of cool stuff that we living things like to do up in the air—like flying! But, for all the good she does us, she can also do some serious damage: tornadoes, hurricanes, and all other manner of nasty storms, for example. If polluted, these storms can become a lot worse, and the air can become near impossible to breathe. Basically, we hardly ever notice our atmosphere, except when we do. But, she’s a majestic, beautiful force of nature who deserves our respect:
Treat her well.
What Makes Earth's Atmosphere Special?
The atmosphere is really important. It is absolutely essential to life as we know it. If you don’t believe me, just check out our two closest planets: Venus, our neighbor on the sun-side of Earth, and Mars, which is farther from the sun than we are.
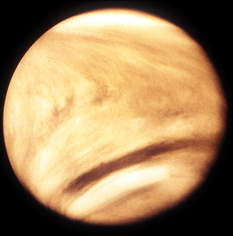
Venus has an atmosphere so dense and full of nasty gases (like carbon dioxide and sulfuric acid) that we can’t even send space probes all the way to the surface without these robots dying. Humans most definitely could not survive there, nor could any other form of life as we know it. This is because of a runaway greenhouse effect that is partially the result of Venus being too close to the hot sun. It’s extreme global warming. If you’re not familiar with the harsh realities of Venus’ atmosphere, this video gives a great introduction:
Venus has an atmosphere so dense and full of nasty gases (like carbon dioxide and sulfuric acid) that we can’t even send space probes all the way to the surface without these robots dying. Humans most definitely could not survive there, nor could any other form of life as we know it. This is because of a runaway greenhouse effect that is partially the result of Venus being too close to the hot sun. It’s extreme global warming. If you’re not familiar with the harsh realities of Venus’ atmosphere, this video gives a great introduction:
Mars, on the other hand, has a very thin, wispy atmosphere. Because Mars is so small (compared to other planets), it just doesn’t have the gravity to hold onto air the way that Earth can (gravity depends on mass). It also doesn’t have all the lifeforms that Earth does, which shape the composition and density of the atmosphere. If humans went there, they wouldn’t be able to get the oxygen they need from the air, and they’d have to wear spacesuits. Mars is also much colder than Earth, partly because its thin atmosphere can’t hold onto heat and partly because it’s so much further from the sun. It’s prone to massive dust storms that last for months—which are also related to the thin atmosphere. If you’re not familiar with the conditions on Mars, this video gives a nice overview:
So, Earth is really right in that Goldilocks-zone for breathable air: not too dense, not too thin, and with just the right combo of atmospheric gases. Here’s a quick comparison of the atmospheres on Venus, Earth, and Mars. Note: all clouds, some clouds, basically no clouds.
What is Earth's Atmosphere Made Of?
Now that we know how special Earth’s atmosphere is, let’s get into the details. What exactly is it made of? And (as a sneak preview of a later sublesson) does it have layers? (Spoiler alert: YES!). What are these layers? And why is our atmosphere the way that it is?
As to our first question: our atmosphere is composed of gas, mainly nitrogen and oxygen. There’s also some other stuff in there, like argon and the trace gases carbon dioxide, neon, helium, methane, nitrous oxide (yes, laughing gas!), and ozone. Trace gases are gases that are only found in very small quantities, less than 1%. This table will give you a breakdown:
As to our first question: our atmosphere is composed of gas, mainly nitrogen and oxygen. There’s also some other stuff in there, like argon and the trace gases carbon dioxide, neon, helium, methane, nitrous oxide (yes, laughing gas!), and ozone. Trace gases are gases that are only found in very small quantities, less than 1%. This table will give you a breakdown:
GasNitrogen
Oxygen
Argon
Carbon Dioxide
Neon
Helium
Methane
Nitrous Oxide
Ozone
|
% Abundance78%
21%
0.9%
0.09%
0.004%
0.001%
0.0004%
0.00008%
0.00001%
|
Don’t memorize that chart. But, you should remember a few very important things about the composition of our atmosphere.
1. The atmosphere is mostly nitrogen: about 80%, versus only about 20% oxygen. Oxygen is the one we breathe. But, it’s actually a good thing that we don’t have too much oxygen in our atmosphere, because:
1. The atmosphere is mostly nitrogen: about 80%, versus only about 20% oxygen. Oxygen is the one we breathe. But, it’s actually a good thing that we don’t have too much oxygen in our atmosphere, because:
- Oxygen is super explosive and our planet probably would have blown up by now if it had more oxygen in the atmosphere. Check out this video of oxygen exploding if you don’t believe me:
- . Too much oxygen can actually be bad for humans (and other life forms). (If you’re curious, too much oxygen results in oxygen toxicity. Symptoms include dizziness, confusion, lip-twitching, severe nausea, spazzy breathing, fainting, blubbering of the lips, seizures, and possible death. It’s related to something called reactive oxygen species in your body, which basically damage everything they can find).
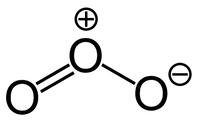
2. There’s only a teensy bit of ozone, but it’s really important. It protects us from harmful UV rays.
If you’re curious, ozone has an interesting chemical structure composed of 3 oxygen atoms, O₃. This is interesting because oxygen-oxygen bonds are super unstable, and this has two of them right next to each other. This unique structure puts the compound at the right energy level to absorb harmful UV light. You don’t need to understand the details of how ozone absorbs UV light for this class, just that it does.
The dumping of nasty stuff (mainly chlorofluorocarbons, a byproduct of plastic manufacturing) into the atmosphere causes thinning of the ozone layer. This means that more of those harmful UV rays get into Earth, which means more sunburned polar bears and more skin cancer for humans, among other concerns. Here’s a video on the ozone layer:
3. There is only a trace amount of carbon dioxide in the atmosphere. We like it that way. We’ll talk a lot more about the implications of having too much carbon dioxide later on, but here’s a sneak-preview:
The summary: Too much carbon dioxide results in global warming/climate change due to a runaway greenhouse effect, just like the one that made Venus completely uninhabitable.
(If you’re a fan of most-likely-fictional sci-fi stories with a moral point, you could also say that, once upon a time, humans lived on Venus, but behaved like us humans today, and caused the complete destruction of the planet that forced them to flee to Earth to survive as a species, just like we will one day flee to Mars. Again, not real science, #FakeNews, but the point is that, if you dump greenhouse gases into the atmosphere, you will eventually destroy the planet in a vicious, irreversible cycle. Don’t be like the Venus people. #ThereArentActuallyVenusPeople #ThatWeKnowOf)
Why Is the Atmosphere The Way It Is?
There are lots of reasons why the atmosphere is the way that it is. We’ll talk about some more of them later on in this lesson. For now, understand reason #1: Earth has just the right amount of gravity to pull in heavier gases, like oxygen and nitrogen, while letting lighter gases, like hydrogen and helium, float away. You can think of a helium balloon floating away from you:
Or a massive blimp of hydrogen gas floating through the sky:
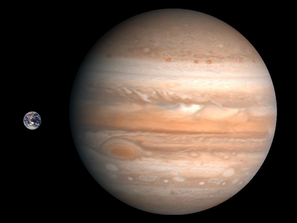
Gravity is related to mass. Compare Earth’s atmosphere to Jupiter’s, which has a really heavy core of ice and rock: as a Gas Giant, Jupiter’s atmosphere is composed of A LOT of hydrogen and helium (that’s what makes it so giant!). That’s because its gravity is strong enough to pull these tiny molecules in.
Note: (a) how big Jupiter is compared to Earth - it is able to pull in so much more gas; and (b) everything we can see of Jupiter is gas. It has a solid core at its center. We’re not totally sure how big the core is, but our best guesses say that it’s at least 10 times as heavy as Earth.
Compare Earth’s atmosphere to Mars’, and you’ll see why it’s so important to life to have enough gravity to be able to hang onto those medium-sized gases like nitrogen and oxygen. Earth’s a pretty special place!
Summary
You should understand:
Hopefully this lesson has given you an appreciation for what is in our atmosphere and why the perfect balance of these things are really important. Be a good citizen of Earth and do what you can to keep the atmosphere just the way it should be, which is perfect for life on earth.
Hint: Good steps that you can take to reduce greenhouse gas emissions include riding a bike, walking, or taking public transportation instead of driving when possible; recycling; reducing the amount of trash that you produce by using reusable products and avoiding packaging when possible; reusing cans, jars, plastic bags, etc.; turning lights off when they’re not in use; driving electric or hybrid vehicles; switching to greener energy sources, like wind, solar, and nuclear power, instead of wood, coal, and fossil fuels; and encouraging schools, businesses, and lawmakers to switch to greener energy sources. This last one is especially important, and there are a lot more ways to help, too!
- How Earth’s atmosphere is special compared to that of Venus and Mars, and how that allows for life on Earth.
- That Earth’s atmosphere is mostly nitrogen, a little ozone, and hopefully only a very small amount carbon dioxide, and why each of these things is important.
- How gravity relates to the composition of a planet’s atmosphere.
Hopefully this lesson has given you an appreciation for what is in our atmosphere and why the perfect balance of these things are really important. Be a good citizen of Earth and do what you can to keep the atmosphere just the way it should be, which is perfect for life on earth.
Hint: Good steps that you can take to reduce greenhouse gas emissions include riding a bike, walking, or taking public transportation instead of driving when possible; recycling; reducing the amount of trash that you produce by using reusable products and avoiding packaging when possible; reusing cans, jars, plastic bags, etc.; turning lights off when they’re not in use; driving electric or hybrid vehicles; switching to greener energy sources, like wind, solar, and nuclear power, instead of wood, coal, and fossil fuels; and encouraging schools, businesses, and lawmakers to switch to greener energy sources. This last one is especially important, and there are a lot more ways to help, too!
Learning Activity
Content contributors: Emma Moulton