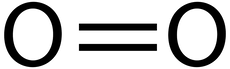Micromolecules
We’ve talked about water, which is hugely important for life and, being only 3 atoms in size, definitely qualifies as a micromolecule, or molecule that is small. But, there are also lots of other really important micromolecules in our bodies and the bodies of other living things, which we’ll talk about in a little bit of detail here.
Micromolecules and Macromolecules
While most of the major functions in our bodies are given to larger molecules (macromolecules), those large molecules could not be built without the small ones, and the large molecules are constantly interacting with the small molecules in order to do their jobs correctly. There are even just single atoms and ions that have important functions in living things. They may be small, yet they are mighty.
We’ve chosen to break down the sublessons into “micromolecules” and “macromolecules,” just for the sake of lumping somewhat similar things together. The actual size cut-off of what makes something a micromolecule versus a macromolecule is a little fuzzy, and the distinction isn’t really that important. The main point we want you to take away is that molecules are responsible for everything that happens in biology, and we want you to understand why some of the key molecules are important and what they do. Once you understand this, you’ll be able to better understand the biological processes we’ll talk about, and you’ll better understand some of the key terms that we’ll use later on.
Without further ado, here are some small yet mighty molecules in the body:
We’ve chosen to break down the sublessons into “micromolecules” and “macromolecules,” just for the sake of lumping somewhat similar things together. The actual size cut-off of what makes something a micromolecule versus a macromolecule is a little fuzzy, and the distinction isn’t really that important. The main point we want you to take away is that molecules are responsible for everything that happens in biology, and we want you to understand why some of the key molecules are important and what they do. Once you understand this, you’ll be able to better understand the biological processes we’ll talk about, and you’ll better understand some of the key terms that we’ll use later on.
Without further ado, here are some small yet mighty molecules in the body:
Oxygen
Oxygen is a simple molecule composed of two oxygen atoms:
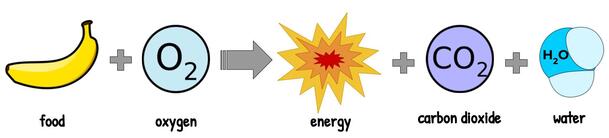
Oxygen is important because it allows us to get as much energy as possible out of the food we eat. More specifically, it is something called an electron donor in a process called cell respiration, which is how our bodies convert sugar into usable energy. We’ll talk more about cell respiration later, so for now just know that oxygen helps us to make energy.
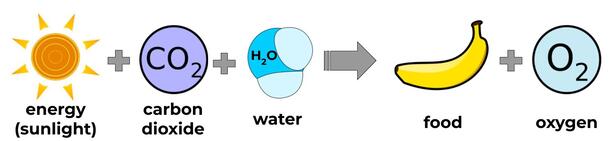
Plants release oxygen as a byproduct (or waste product) of photosynthesis, which is the process that turns energy from the sun (along with carbon dioxide and water) into food (sugar). That’s really handy for us because it gives us something to breathe, but it doesn’t mean plants don’t need oxygen. They still do cell respiration, so they still need oxygen to help them get usable energy from their food.
Strictly speaking, though, not all living things need oxygen. When we talk about cell respiration, we’ll learn that there’s a step that makes some energy and doesn’t use any oxygen. Some species, especially bacteria, live just on that energy and don’t need any oxygen. There are even cells in our bodies (our muscles!) that can survive without oxygen for a little bit using just that other little bit of energy. This is useful when we’re exercising really hard and using oxygen faster than we’re taking it in.
Here’s a great video on why we need oxygen:
Here’s a great video on why we need oxygen:
Carbon Dioxide
We breathe out carbon dioxide as a waste product, but, just like plants give off oxygen but still need that, we still need carbon dioxide! Carbon dioxide acts as a source of carbon that helps us to build other carbon-containing things, which is most things in our body.
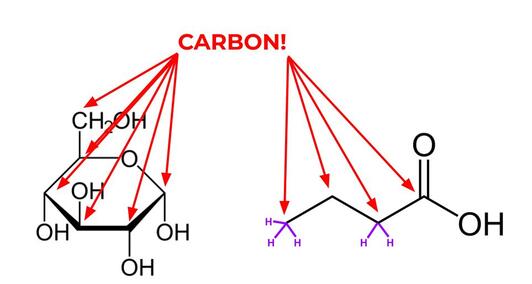
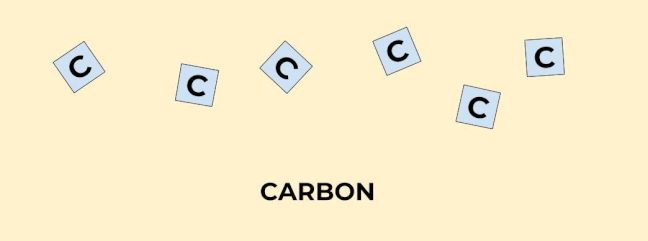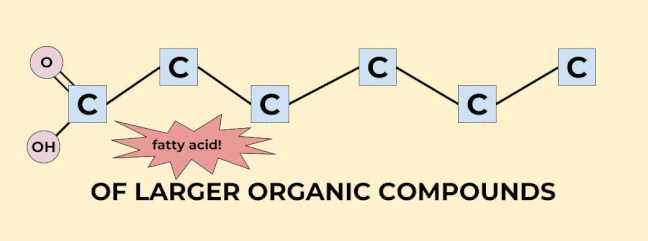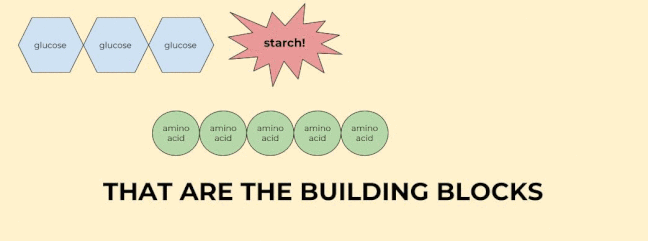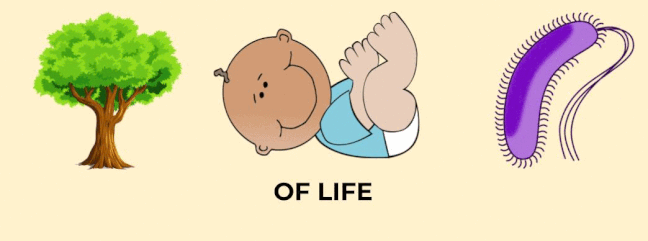
Carbon is super important--just about every molecule in our body (and the bodies of other living things) contains carbon! In fact, something is only considered an organic molecule if it contains carbon and comes from our bodies. Organic molecules are the building blocks of life. (I know we said that cells are the building blocks of life, and that is true, but molecules are the building blocks of cells, so… point is, they’re important). You can think of carbon dioxide like little Lego pieces that fit together to make big organic compounds, like sugar and starch. It’s not exactly like that, but that’s the general idea. Note that this use of organic is different from the one you might have heard about when people are talking about food, which means that no pesticides were used in the process of growing the food.
Carbon is super important--just about every molecule in our body (and the bodies of other living things) contains carbon! In fact, something is only considered an organic molecule if it contains carbon and comes from our bodies. Organic molecules are the building blocks of life. (I know we said that cells are the building blocks of life, and that is true, but molecules are the building blocks of cells, so… point is, they’re important). You can think of carbon dioxide like little Lego pieces that fit together to make big organic compounds, like sugar and starch. It’s not exactly like that, but that’s the general idea. Note that this use of organic is different from the one you might have heard about when people are talking about food, which means that no pesticides were used in the process of growing the food.
|
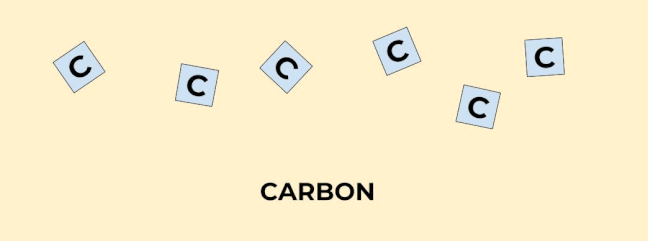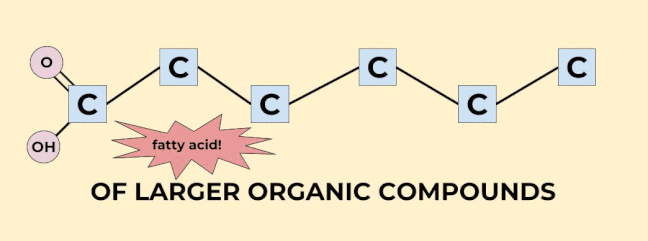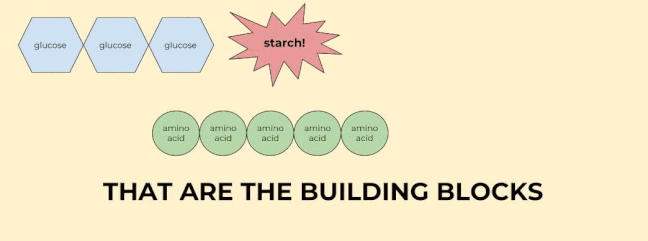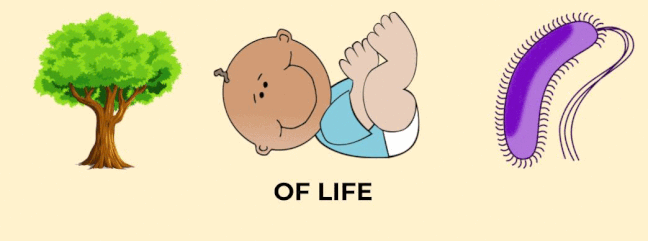
Carbon is so important for life that we don’t even have to use a “C” to indicate a carbon in an organic molecule. Any corner or end (that is, anything with a bond coming off of it that isn’t indicated by a letter) in an organic molecule is just known to be carbon. (Well, carbon with some hydrogens coming off of it. Hydrogen is so important that we don’t have to draw it on carbon at all, we just know that carbon has as many hydrogens on it as it takes to make 4 bonds. I’ve drawn a couple of hydrogens on the organic molecules shown above just to give you the idea, but every single carbon has hydrogens on it! Imagine being so important that you can be completely invisible and everyone still knows you’re there!). |
dioxide is especially important for plants and bacteria that do photosynthesis, because it is the source of carbon that allows them to make sugar from light energy. They then use this sugar to make usable energy for themselves, and then store the rest for later. When we eat vegetables--especially the starchy ones like potatoes, carrots, and beets--as well as fruits, we’re eating this stored sugar.
|
The actual chemistry is unfortunately not this simple, but: Carbon (in the form of carbon dioxide) is the building block of larger organic compounds that are the building blocks of life. To make it even easier: Carbon is the building block of life!
|
In complex animals (like humans!), carbon dioxide also acts as a buffer in the blood, which means it helps us keep our pH steady (right around 7.4). If you’re curious, this is how the bicarbonate buffer system works. You don’t need to remember the details for this class—just know that carbon dioxide is involved in keeping blood pH steady:
Electrolytes
We call the most important ions in our bodies electrolytes. These ions include sodium (Na⁺), potassium (K⁺), chloride (Cl⁻), calcium (Ca²⁺), and magnesium (Mg²⁺). When a lot of ions move together in one direction, it creates an electrical current.
Electrical currents are responsible for all kinds of electricity in the world around us, including the electricity in your lightbulbs and in your computer (batteries, on a chemical level, are filled with electrolytes--but dangerous ones, not ones we want in our bodies). In our bodies, we use electricity in the form of moving electrolytes to help us send nerve impulses, like the ones our brain uses to think, feel, and tell our bodies to move. Moving ions also cause the electrical currents that cause our muscles to move, our hearts to beat, and our diaphragm (breathing muscle) to pull in air. Moving ions can also be involved in bringing nutrients into the cells that need them.
Electrical currents are responsible for all kinds of electricity in the world around us, including the electricity in your lightbulbs and in your computer (batteries, on a chemical level, are filled with electrolytes--but dangerous ones, not ones we want in our bodies). In our bodies, we use electricity in the form of moving electrolytes to help us send nerve impulses, like the ones our brain uses to think, feel, and tell our bodies to move. Moving ions also cause the electrical currents that cause our muscles to move, our hearts to beat, and our diaphragm (breathing muscle) to pull in air. Moving ions can also be involved in bringing nutrients into the cells that need them.
|
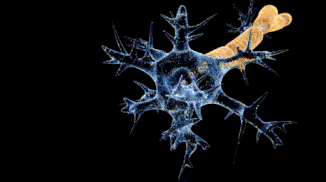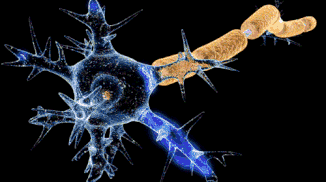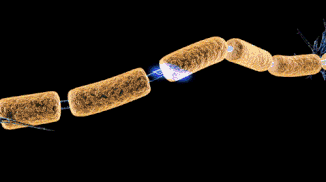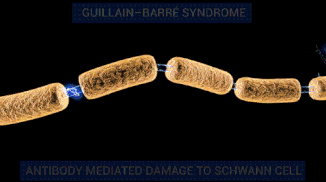
That funny-looking, star-shaped thing is a nerve cell (like in your brain, in your spinal cord, and connected to your muscles), and the bolt of lightning running down it is an electrical current. That electrical current is a signal that allows nerve cells to talk to each other and to other cells, like muscles. This signal is caused by ions.
|
All of the exact ways that our bodies use electrolytes can get a little complicated, and we’ll talk about a few of them in more detail later. For now, know that ions create electrical signals that are really important for our bodies, especially our nerves and muscles.
Vitamins
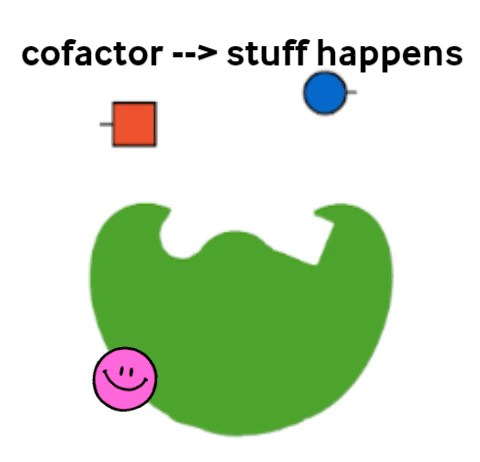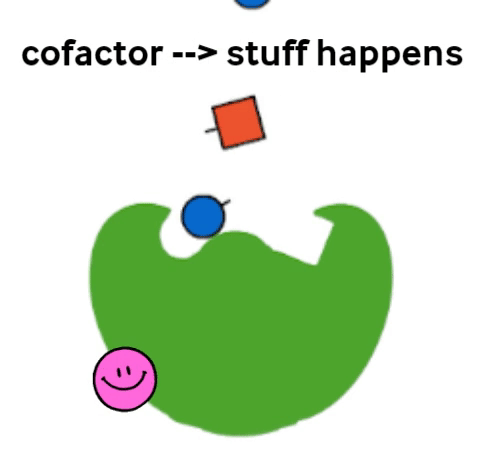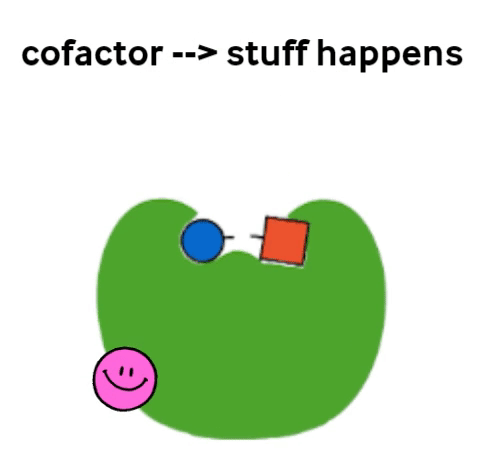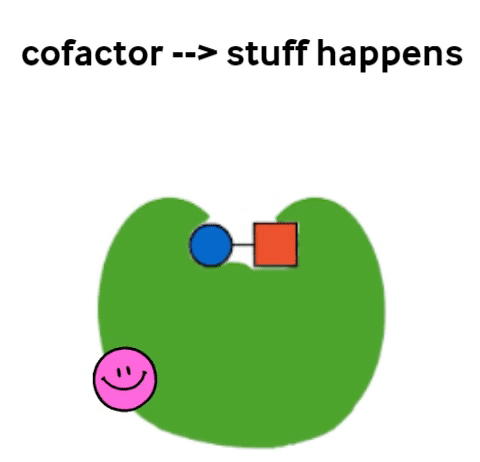
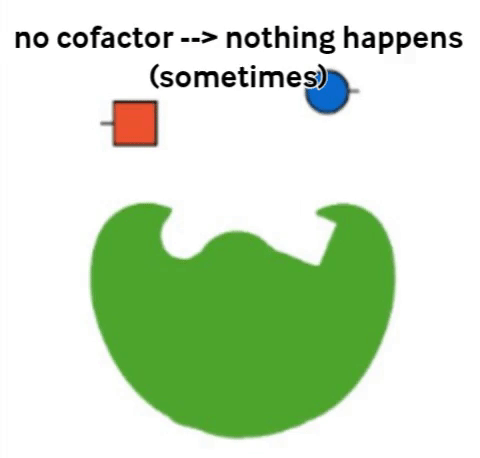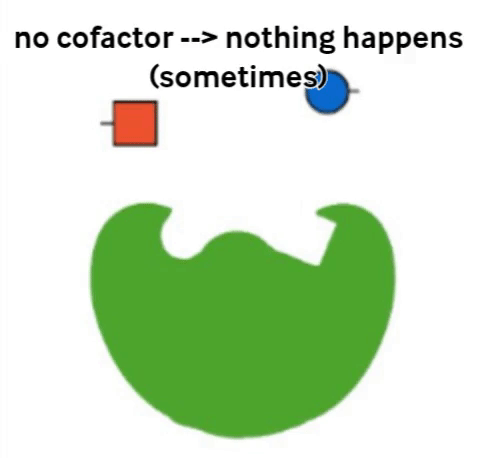
Vitamins are what are called cofactors in many chemical reactions in our bodies. That means that they don’t actually have their own job, but they help other, bigger molecules (called enzymes) to do their job. Not all enzymes need a vitamin to do their jobs, but all vitamins help an enzyme to do its job.
If you’d like to learn more about vitamins, this video gives a great overview:
Monomers
Last but certainly not least, monomers are the building blocks of polymers, which are, by definition, many monomers linked together. I know that’s kind of a roundabout definition, but basically it means that monomers are any chemical structures that can be linked together in a chain.
Yes, I know I put this in here already. I wonder if it’s important? (Spoiler! It is.)
The macromolecules that we will talk about later in this lesson are all polymers.
The key monomers in our bodies are glucose (sugar), amino acids, fatty acids, and nucleic acids. We’ll talk more about these monomers when we talk about their polymers.
The key monomers in our bodies are glucose (sugar), amino acids, fatty acids, and nucleic acids. We’ll talk more about these monomers when we talk about their polymers.
Summary
You’ve might have heard of most of these molecules before, and that’s because they’re really important for life. That’s pretty much the point of biochemistry: to explain the molecules that are important for life. Our goal here is to shed some light on why these molecules are important and what they do, so that you can better understand your body and the world around you. That is a really cool thing to learn about! It can also be super useful. Once we understand how stuff works, we can start to understand how we might be able to hijack it for useful purposes, like medicine or improving agriculture. This is just the beginning. While you may not see the immediate usefulness just yet, the foundations of knowledge that you learn here will help you understand much more complicated processes and, eventually, allow you to use science for good. Get excited!
You should understand:
You should understand:
- The key functions of these molecules:
- Oxygen: Important in cell respiration, which is the cellular process that allows us to make usable energy
- Carbon dioxide: The building blocks of larger organic molecules (which are the building blocks of even bigger organic molecules, which are the building blocks of cells, which are the building blocks of life). Also important in maintaining blood pH.
- Electrolytes: create electrical signals like nerve impulses and muscle contractions
- Vitamins: Help proteins do their job right
- Monomers: Make polymers (macromolecules)
Learning Activity
Content contributors: Emma Moulton