Diffusion
As you now know, particles such as atoms and molecules are the building blocks of matter, and everything around you—papers, your computer, water, etc.—is made up of these particles. Even you are made up of them.
But how do these particles behave? How do they interact with each other?
Well, as you’ve also learned, particles are always moving around. They’re like little kids hopped up on sugar. They collide with each other and spread out. They move faster when we heat them up and slower when we cool them down. In this lesson, we will be looking at a particular way in which molecules move, called diffusion. In order to fully discuss the idea of diffusion, we will also introduce solution chemistry.
But how do these particles behave? How do they interact with each other?
Well, as you’ve also learned, particles are always moving around. They’re like little kids hopped up on sugar. They collide with each other and spread out. They move faster when we heat them up and slower when we cool them down. In this lesson, we will be looking at a particular way in which molecules move, called diffusion. In order to fully discuss the idea of diffusion, we will also introduce solution chemistry.
Diffusion: Molecules Spreading Out
Have you ever sprayed air freshener into a room and watched as the mist “disappeared” into the air? Or put food coloring into a drink and watched as the whole drink turned into a single color after a while?
You may have also noticed that, after spraying the air freshener and leaving the room for a few minutes, the entire room smells like the freshener, regardless of where you are inside of it. These real-life situations demonstrate exactly what happens in the process of diffusion.
What is Diffusion?
Diffusion is the net (overall) movement of a substance from a region of high concentration to a region of low concentration. Basically, it means that when there’s a lot of something in one area, it will spread out to become less crowded. It’s like how people generally don’t stay in a giant crowd in the corner of the room: they spread out to fill the space that they have.
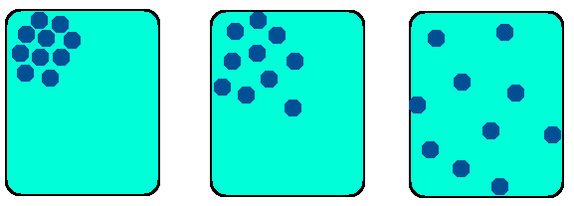
Diffusion on a molecular level: they start off close together, and move to become further apart
When molecules move to spread out from each other, we say that they are moving down their concentration gradient. A concentration gradient is simply a difference in concentration between two areas—one is crowded, the other is less so. We say “down” because being close to other molecules is high energy, and being far from other molecules is low energy. Like sliding down a slide. Because of this energy difference, and because things love to be low in energy, diffusion happens naturally down a concentration gradient. In other words, diffusion only occurs from high concentrations to low concentrations.
Diffusion continues until equilibrium is reached. Equilibrium is a state of balance where there is no net change. It just stays steady and equal and the same.
To reiterate this idea, just in case you got tripped up in the vocabulary: molecules want to be less crowded.
Diffusion continues until equilibrium is reached. Equilibrium is a state of balance where there is no net change. It just stays steady and equal and the same.
To reiterate this idea, just in case you got tripped up in the vocabulary: molecules want to be less crowded.
Brownian Motion and Diffusion
The ability for molecules to diffuse at all comes back to a concept called Brownian motion. Brownian motion is simply the random movement of particles. It looks like this:
This random movement leads to collisions. Molecules constantly bump into things in more crowded areas (there are more collisions), and these collisions cause them to move. Think of someone stumbling backwards when you bump into them. Molecules will keep colliding until they are far away from the stuff they are colliding with. Eventually, everything will become spread out. They will stay that way because they are less likely to be “bumped backwards” in more crowded areas.
Dispersion
There is also a related concept called dispersion, but it’s important not to confuse them. It is similar to diffusion in that it causes molecules to spread apart until they are uniform (evenly dispersed). But, it is not linked to Brownian motion. Rather, it is linked to mechanical forces, like stirring. Yellow dye will spread through water because of dispersion just like it would with diffusion, but it will go faster if we shake or stir the water (depending on your preferences, Mr. Bond).
Summary
This video does a great job of explaining diffusion, and also gives you a sneak peak at some of the biological implications:
Now let’s test your understanding. If there are 10 particles on one half of a room and 0 on the other, in what (net) direction does diffusion occur?
Because particles move down a concentration gradient, they move from high concentrations to low concentrations. Accordingly, the particles would move away from the side with the 10 particles, and towards the side with no particles, until there are 5 particles on each side (a perfect balance, or equilibrium).
You should understand:
- That, in the process of diffusion, molecules naturally move down their concentration gradients from areas of high concentration to low concentration, until they reach equilibrium, which is a state of balance where there is no net change.
- That molecules are constantly moving in random patterns, which is called Brownian motion.
- The difference between dispersion, which is mixing due to mechanical forces like shaking and stirring, and diffusion, which is a natural process that occurs due to collisions in Brownian motion.
Learning Activity
Content contributors: Connie Chen, Emma Moulton