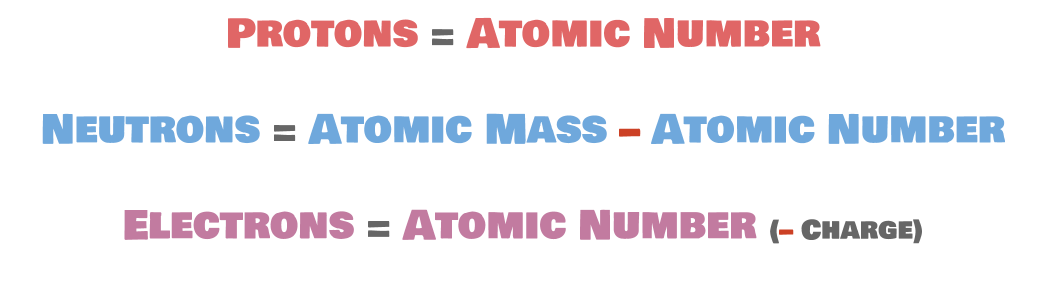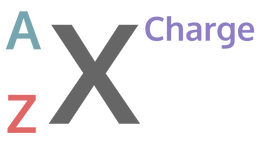Modeling Atoms
Why Do We Need Models?
Imagine that you have gone back in time to the early 1800's, long before the dawn of technology. Now imagine trying to explain to them what a car is. What do you tell them? You could say that it has an engine and a place for people to sit, you could describe a steering wheel to them, and you could explain how it gets you places faster than walking would. You ask them to draw it back for you, and this is what you get:
Probably not quite what you had in mind, huh? Wouldn't it just have been easier to show them a picture of a car? The same goes for science, especially when it comes to atoms. Everything in science becomes so much easier when we can see how things work. But how can we see how atoms work when atoms are so small?
The simple answer is that we use models. Models represent ideas as pictures. Models don't necessarily look exactly like the thing they describe: Instead, they're designed to give us useful information and conceptualization about the thing we're describing. For example, in our car example, one useful model would be a blueprint. A blueprint isn't an exact model of the car, but it gives you even more information about it than a picture might, such as by including the location and the parts of each major engine component, safety feature, and so on, as well as the relative dimensions of those items to each other. This is a very useful model that gives us a lot of information.
Two of the most fundamental, useful models that we use in Chemistry to describe atoms and their important features are atomic symbols and the Bohr model. What are these exactly, and how do we use these über-awesome models, you ask? Let's take a look.
The simple answer is that we use models. Models represent ideas as pictures. Models don't necessarily look exactly like the thing they describe: Instead, they're designed to give us useful information and conceptualization about the thing we're describing. For example, in our car example, one useful model would be a blueprint. A blueprint isn't an exact model of the car, but it gives you even more information about it than a picture might, such as by including the location and the parts of each major engine component, safety feature, and so on, as well as the relative dimensions of those items to each other. This is a very useful model that gives us a lot of information.
Two of the most fundamental, useful models that we use in Chemistry to describe atoms and their important features are atomic symbols and the Bohr model. What are these exactly, and how do we use these über-awesome models, you ask? Let's take a look.
Atomic Symbols
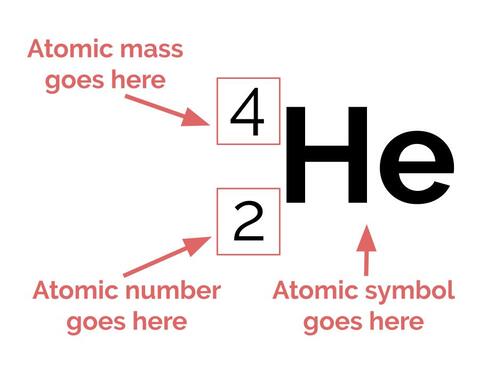
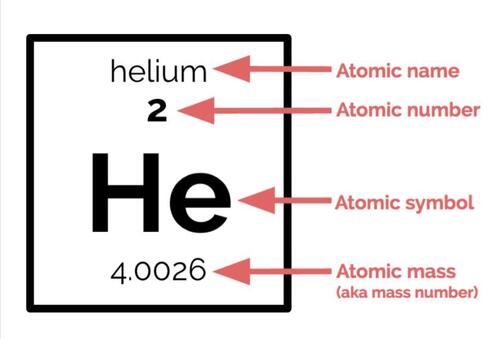
One method that scientists use to describe atoms is the atomic symbol. Even though it is not very elaborate, it is very helpful in conveying basic information about a particular element: namely, the atomic number, the atomic mass, and the charge. This is what an atomic symbol looks like:
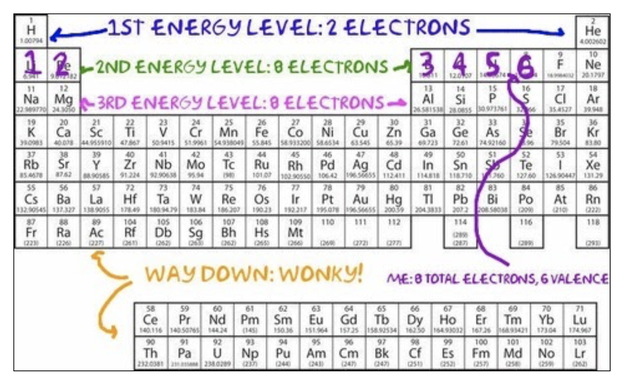
All of the information that you need to write these atomic symbols is contained in the Periodic Table, your new best friend:
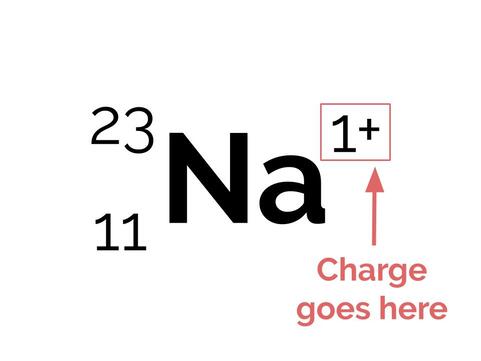
Some atoms have a charge: We’ll talk about this more soon. If an atom is charged, we simply put it to the top right of the atomic symbol, like so:
Why is it useful to know the atomic mass, the atomic number, and the charge? Well, aside from the fact that each of these features is useful in telling us about some baseline properties of the atom, they can also tell us how many protons, neutrons, and electrons an atom has.
We've already learned about atomic number and atomic mass. As a reminder, the atomic number is the number of protons in an atom of a particular element. Hydrogen, which has an atomic number of 1, has one proton in the nucleus. Oxygen, which has an atomic number of 8, has 8 protons in its nucleus.
Atomic mass is the overall mass (weight) of the atom in amu. When putting it into an atomic symbol, we round to the nearest whole number. Because both protons and neutrons have a mass of about 1 amu, and electrons have negligible mass, atomic mass is roughly equal to the number of protons plus the number of neutrons. We can use this information combined with atomic number to figure out exactly how many neutrons is in an atom.
Let's see an example: Hydrogen, which has an atomic number of 1, has 1 proton in its nucleus. It also has an atomic mass of about 1 (it's actually 1.0079, but close enough). Can you figure out how many neutrons is in the nucleus?
...
...
Hydrogen has zero neutrons in its nucleus! Protons + neutrons = atomic mass. Protons = 1. Atomic mass = 1. 1 + neutrons = 1, so neutrons must equal 0.
Can you figure out how many protons and neutrons oxygen has? Oxygen has an atomic mass of 15.999 and an atomic number of 8.
...
...
Oxygen has 8 protons and 8 neutrons! Atomic number = protons = 8, and atomic mass (16) = protons (8) + neutrons, so neutrons must equal 8. (Don't worry about why the atomic mass isn't exactly a whole number for this class. If you really want to know, it has to do with there being different naturally occurring radioactive isotopes, which have different numbers of neutrons. We're only concerned with the most common isotopes in this class, which is the one you get from rounding to the nearest whole number.)
Now onto electrons! In an uncharged element—anything that's not an ion—the number of electrons is exactly the same as the number of protons. This is just to do with the concept of what it means to have a charge. If something is charged, then the total number of + charges is not the same as the number of – charges. If there are more + charges than – charges, there is an overall + charge. If there are more – charges than + charges, then there is an overall – charge. So, if there are more protons than electrons, there is an overall + charge (if there are two more protons than electrons, there is a 2⁺ charge, if there are three more protons than electrons, there is a 3⁺ charge, and so on), and if there are more electrons than protons, then there is an overall – charge (or 2⁻, or 3⁻, etc.).
Back to atoms without charges: the number of electrons is the same as the number of protons, which is the same as the atomic number. Helium, which has an atomic number of 2, has two protons and two electrons. We'll talk about ions and charges again when we talk about ionic bonding. For now, focus on finding the number of electrons for uncharged elements.
To review:
We've already learned about atomic number and atomic mass. As a reminder, the atomic number is the number of protons in an atom of a particular element. Hydrogen, which has an atomic number of 1, has one proton in the nucleus. Oxygen, which has an atomic number of 8, has 8 protons in its nucleus.
Atomic mass is the overall mass (weight) of the atom in amu. When putting it into an atomic symbol, we round to the nearest whole number. Because both protons and neutrons have a mass of about 1 amu, and electrons have negligible mass, atomic mass is roughly equal to the number of protons plus the number of neutrons. We can use this information combined with atomic number to figure out exactly how many neutrons is in an atom.
Let's see an example: Hydrogen, which has an atomic number of 1, has 1 proton in its nucleus. It also has an atomic mass of about 1 (it's actually 1.0079, but close enough). Can you figure out how many neutrons is in the nucleus?
...
...
Hydrogen has zero neutrons in its nucleus! Protons + neutrons = atomic mass. Protons = 1. Atomic mass = 1. 1 + neutrons = 1, so neutrons must equal 0.
Can you figure out how many protons and neutrons oxygen has? Oxygen has an atomic mass of 15.999 and an atomic number of 8.
...
...
Oxygen has 8 protons and 8 neutrons! Atomic number = protons = 8, and atomic mass (16) = protons (8) + neutrons, so neutrons must equal 8. (Don't worry about why the atomic mass isn't exactly a whole number for this class. If you really want to know, it has to do with there being different naturally occurring radioactive isotopes, which have different numbers of neutrons. We're only concerned with the most common isotopes in this class, which is the one you get from rounding to the nearest whole number.)
Now onto electrons! In an uncharged element—anything that's not an ion—the number of electrons is exactly the same as the number of protons. This is just to do with the concept of what it means to have a charge. If something is charged, then the total number of + charges is not the same as the number of – charges. If there are more + charges than – charges, there is an overall + charge. If there are more – charges than + charges, then there is an overall – charge. So, if there are more protons than electrons, there is an overall + charge (if there are two more protons than electrons, there is a 2⁺ charge, if there are three more protons than electrons, there is a 3⁺ charge, and so on), and if there are more electrons than protons, then there is an overall – charge (or 2⁻, or 3⁻, etc.).
Back to atoms without charges: the number of electrons is the same as the number of protons, which is the same as the atomic number. Helium, which has an atomic number of 2, has two protons and two electrons. We'll talk about ions and charges again when we talk about ionic bonding. For now, focus on finding the number of electrons for uncharged elements.
To review:
This video provides a great tutorial on writing atomic symbols, if there's still any confusion:
The Bohr Model
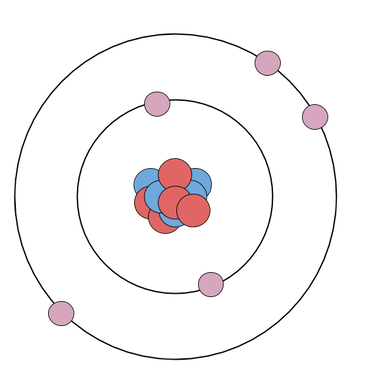
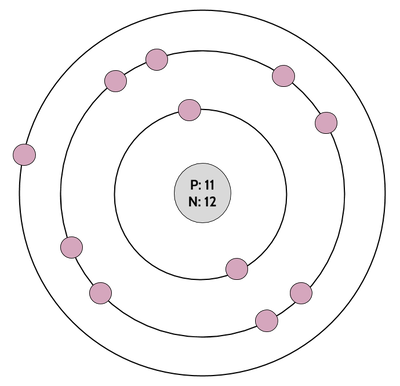
The Bohr model is a bit of an icon in Chemistry. Like, kinda a mega-celebrity. I mean, just look at this beautiful heartthrob:
I mean, just oooohhhh!! So pretty. I mean just look at that guy! I'd give it my heart and soul. *Faints*
Okay, but, for reals, the Bohr model is really useful because it shows us at a quick glance the number of valence electrons that an atom has, which tells us how easily the atom will react and form chemical bonds. As you may recall from previously, valence electrons are the electrons in the outer shell (here, the outer ring). With the exception of ring number 1 (the innermost ring), which gets full with 2 electrons, and some super-way-out-there rings that we won't worry about in this class, atoms like to have 8 electrons in their valence shell. Atoms are most stable when they have a full valence shell. That basically means that they will keep reacting and keep bonding until they get their full valence shells. We'll talk more about this when we talk about chemical bonding. First and foremost, in order to be able to use this awesome tool for chemical bonding, we need to know how to draw Bohr models.
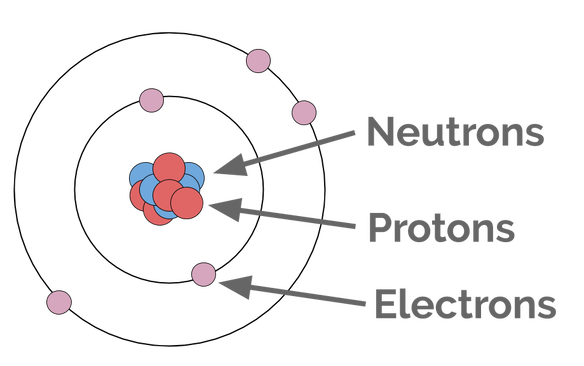
Let's break down the parts of the Bohr model:
Okay, but, for reals, the Bohr model is really useful because it shows us at a quick glance the number of valence electrons that an atom has, which tells us how easily the atom will react and form chemical bonds. As you may recall from previously, valence electrons are the electrons in the outer shell (here, the outer ring). With the exception of ring number 1 (the innermost ring), which gets full with 2 electrons, and some super-way-out-there rings that we won't worry about in this class, atoms like to have 8 electrons in their valence shell. Atoms are most stable when they have a full valence shell. That basically means that they will keep reacting and keep bonding until they get their full valence shells. We'll talk more about this when we talk about chemical bonding. First and foremost, in order to be able to use this awesome tool for chemical bonding, we need to know how to draw Bohr models.
Let's break down the parts of the Bohr model:
You already know how to calculate the number of protons, neutrons, and electrons (check out that learning!), so next we just need to look at how they're arranged.
Protons and neutrons go in the nucleus. That's not really the interesting part here, and drawing a lot of protons and neutrons is cumbersome, so we often just simplify that bit like this:
Protons and neutrons go in the nucleus. That's not really the interesting part here, and drawing a lot of protons and neutrons is cumbersome, so we often just simplify that bit like this:
Now comes the rub. (For those of you who do not frequent Shakespeare or Pirates of the Caribbean, that would be British slang for "the tricky part").
Electrons go in "shells," which are drawn like concentric circles, around the nucleus. The first shell gets full when it has 2 electrons. All the other shells (that we use in this class) get full when they have 8 electrons. We call this a full octet. The Periodic Table helps us to count:
Electrons go in "shells," which are drawn like concentric circles, around the nucleus. The first shell gets full when it has 2 electrons. All the other shells (that we use in this class) get full when they have 8 electrons. We call this a full octet. The Periodic Table helps us to count:
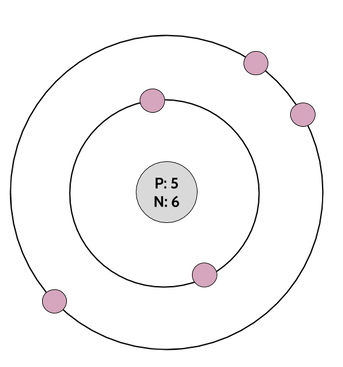
So, once we've figured out the number of electrons, all we have to do is draw them in, with 2 in the inner circle (or just 1 if the atom only has one electron in total, like hydrogen), and the rest filling up the other circles as they go out. For boron (drawn above), that means 3 electrons go in the second shell (for 5 electrons total). For sodium, which has 11 electrons, that would look like this:
Simple as! Guess it's not too much of a "rub" after all...
This video provides a good tutorial, if you want to go through that again (you can ignore the bit on electron configuration):
This video provides a good tutorial, if you want to go through that again (you can ignore the bit on electron configuration):
Summary
You should understand:
- How to write an atomic symbol, with the correct placement of the atomic number, atomic mass, and (if applicable) charge.
- How to determine the number of protons, neutrons, and electrons that an element contains, based on the information in the Periodic Table.
- How to draw a Bohr model, including the correct placement of protons, neutrons, and electrons.
Learning Activity
The best way to learn any new skill is with a lot of practice. So, we'll have you draw out a bunch of atomic symbols and Bohr models on your own. You can then input into the forms below to check your answers.
We recommend that you draw out the atomic symbols and draw the Bohr models indicated in the form and then use that information to input your answers. To simplify checking your atomic symbols, we'll have you put in what A, X, and Z were, based on these definitions of A, X, and Z:
We recommend that you draw out the atomic symbols and draw the Bohr models indicated in the form and then use that information to input your answers. To simplify checking your atomic symbols, we'll have you put in what A, X, and Z were, based on these definitions of A, X, and Z:
Contributors: Emma Moulton and Emily Zhang