Semi-Permeable Membranes
Diffusion and Osmosis Across a Semi-permeable Membrane
Now that we’ve defined diffusion in a general sense and learned to quantify the concentration of solutions using molarity, we can apply the concept of diffusion to systems involving a semipermeable membrane. These are really just examples of what you have already learned.
What is a Semi-permeable Membrane?
A semipermeable membrane is a membrane, or thin layer (such as a thin piece of plastic or a cell membrane, which is a biology thing that we will talk about later), that only lets certain things cross it. For example, it could have little tiny holes that only let very small things through them, or it could have chemical properties that don’t let certain things cross. So, they might let water cross, but not solutes.
So, why do we care about semi-permeable membranes? Mostly because they are how the tiny cells in our bodies (and the bodies of all living things) decide what gets to cross them by using a semi-permeable membrane (the cell membrane). It is made up of molecules called phospholipids that only let certain things, like oxygen and water, cross them, while excluding other things, like poison. When we get to the biology section of this class, we’ll talk a lot more about cells and cell membranes.
This video provides a great overview of why selective permeability is important to cell membranes:
So, why do we care about semi-permeable membranes? Mostly because they are how the tiny cells in our bodies (and the bodies of all living things) decide what gets to cross them by using a semi-permeable membrane (the cell membrane). It is made up of molecules called phospholipids that only let certain things, like oxygen and water, cross them, while excluding other things, like poison. When we get to the biology section of this class, we’ll talk a lot more about cells and cell membranes.
This video provides a great overview of why selective permeability is important to cell membranes:
What is Osmosis, and When Does it Occur?
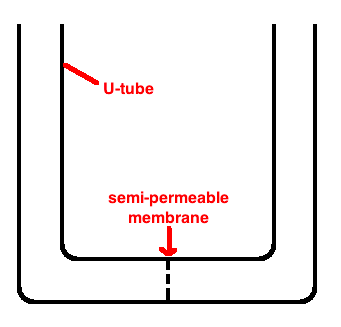
For now, to make things simple, we’ll set aside the cell membrane and explain how diffusion and osmosis work using a U-tube. Osmosis is the diffusion of water: more on this in a second. A U-tube is mainly just a model that we use to understand the process better. A U-tube looks like this:
It is useful because we can put two solutions of different concentrations on either side of the membrane and see what happens. Here’s a YouTube video of a U-tube at work:
If the membrane lets the solutes across, then diffusion will occur until they are of equal concentrations (this also means both sides will be of equal molarity). This is exactly what you have already learned about diffusion.
If the membrane doesn’t let solutes across, then water will move instead. This is called osmosis. Osmosis works just like diffusion: water moves from areas where water is most concentrated to areas where water is least concentrated. Don’t let this trip you up: if water is more concentrated, then the solution is actually more dilute (it has less solute). The same principle applies to a concentrated solution: more solute means relatively less water, in a given volume. A useful way to think about this: in a fixed volume, you only get a certain amount of solute and water. If solute goes down, water must go up. If solute goes up, water must go down. Osmosis goes from where water is high to where water is high.
This video give a great overview of osmosis:
Let’s check your understanding: does water move from high concentration of solute to low concentration of solute or low to high?
Low to high! Even though this might seem like the opposite of what I’ve told you about diffusion, it is really the exact same principle.
Solving U-Tube Problems About Osmosis and Diffusion
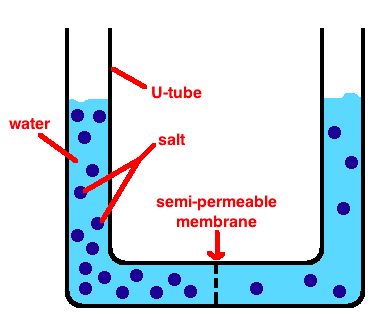
To see osmosis in practice, let’s go back to our U-tube. Let’s fill the left side with a high concentration of salt and the right side with a low concentration of salt. They will be in solution with water.
Hopefully you can see that we have just made a concentration gradient. In other words, solute wants to spread out (diffuse) across the membrane until both sides have equal concentrations of salt. And, if our membrane lets the salt cross, that is exactly what will happen.
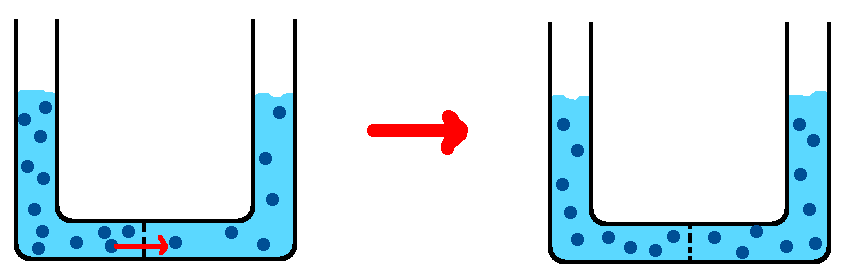
Can you draw a picture of what diffusion of salt across this membrane would look like?
…
...
If you drew something like this, you understand diffusion pretty well!
Can you draw a picture of what diffusion of salt across this membrane would look like?
…
...
If you drew something like this, you understand diffusion pretty well!
The total number of solute molecules is the same, but they have spread out so that the number of molecules on each side of the tube is equal.
Note that, since we have changed the number of moles of salt on each side of the tube, the molarity on each side of the tube is different than it was before, and now the molarity on both sides is the same as each other.
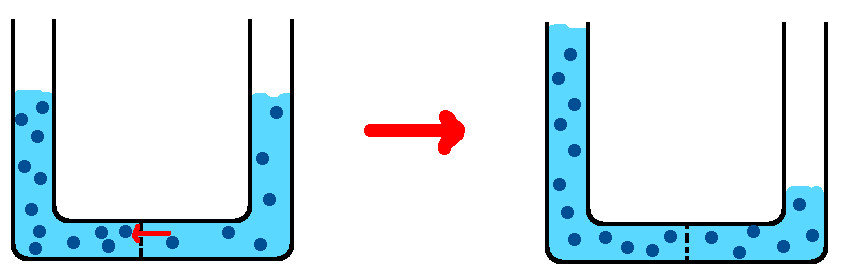
But, now look what happens if the membrane is impermeable to salt.
Note that, since we have changed the number of moles of salt on each side of the tube, the molarity on each side of the tube is different than it was before, and now the molarity on both sides is the same as each other.
But, now look what happens if the membrane is impermeable to salt.
Since the salt couldn’t move like it wanted to, the water moved instead. Water’s an awesome go-getter, problem-solver that way. Water moves by osmosis to make the concentrations equal. Even though the amounts of solute and water are different on each side of the tube, the concentrations are equal, and that’s what matters. (Concentration is how spaced out the salt molecules are).
Note that the number of salt molecules on each side of the tube is the same as it was to begin with. But, since the amount of water has been changed (the liters of solvent), the molarity is still equal on both sides, just like with regular diffusion.
One final thought to keep in mind: water is always moving back and forth across a membrane. The same applies to all solutes that can cross the membrane. We are trying to figure out what the net (overall) movement is.
Note that the number of salt molecules on each side of the tube is the same as it was to begin with. But, since the amount of water has been changed (the liters of solvent), the molarity is still equal on both sides, just like with regular diffusion.
One final thought to keep in mind: water is always moving back and forth across a membrane. The same applies to all solutes that can cross the membrane. We are trying to figure out what the net (overall) movement is.
Summary
This video gives a great overview of the most important concepts in diffusion and osmosis:
You should understand:
- What diffusion and osmosis are, how they are the same, how they are different, and why osmosis would occur instead of diffusion.
- The direction of water movement during osmosis and how this relates to solute concentration.
- How to determine the direction of movement of solutes or water across a semipermeable membrane in a U-tube, both when solutes are allowed across and when they aren’t.
- That semipermeable membranes have important implications in biology.
Learning Activity
Content contributors: Emma Moulton and Emily Zhang