EIS 17: Biochemistry
Activity 1: Canned Fire
Caution: This experiment involves fire. Always get the permission of your relevant responsible adult before doing any experiments involving fire. Use caution when handling fire. It is recommended that you keep a fire extinguisher handy, just in case.
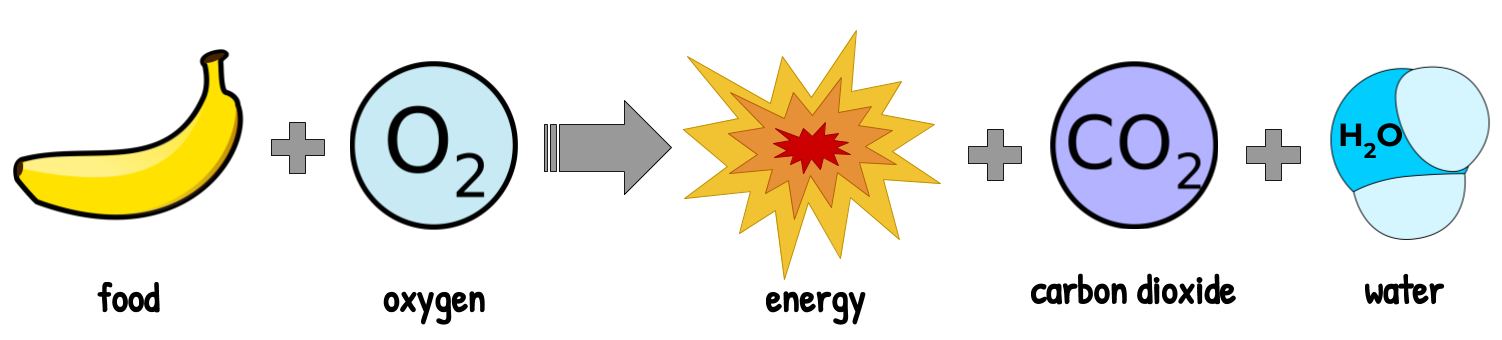
In this lesson, we learned that different types of macromolecules store different amounts of energy, which is released during the process of cell respiration:
In this lesson, we learned that different types of macromolecules store different amounts of energy, which is released during the process of cell respiration:
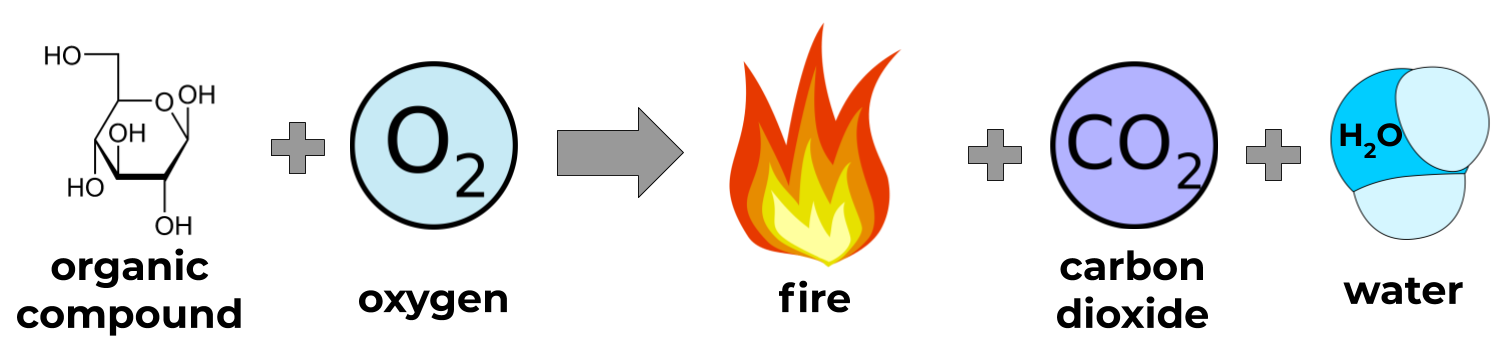
Cell respiration is really just a controlled combustion reaction. A combustion reaction is a reaction in which an organic compound interacts with oxygen to release energy, water and carbon dioxide:
We’ll learn later that cell respiration gradually releases the same energy from a combustion reaction, but instead collects it at every step to turn it into useful energy.
Because the processes that make energy in our bodies are just combustion reactions, we can compare the amount of energy that comes out of different food by burning it, which is the goal of this activity.
Because the processes that make energy in our bodies are just combustion reactions, we can compare the amount of energy that comes out of different food by burning it, which is the goal of this activity.
Materials
- 3 aluminum cans. Rinsed out soup/bean cans work great, as do soda cans with the tops cut off. You can also use candle tins or make a shallow dish/“boat” out of aluminum foil, but use extra caution as the fire will be exposed.
- 1 tbsp granulated sugar.
- 1 tbsp olive oil. Most other kitchen oils will also work.
- 1 tbsp protein powder. If you do not have protein powder, you can skip this one. Unsweetened is best.
- 3 tsp rubbing alcohol. Any concentrated alcohol will work.
- Matches (recommended) or a barbeque lighter (the kind with a long nozzle).
- Stopwatch. An electronic one, like on your phone, will work.
- Pot lid or ceramic plate.
- Fire extinguisher (recommended) or a fire blanket or damp towel (that you don’t care about).
Procedure
- Put 1 tbsp granulated sugar into the first tin, 1 tbsp olive oil in the second, and 1 tbsp protein powder in the third.
- Add 1 tsp rubbing alcohol to tin #1.
- Start your stopwatch as you drop a lit match (or light using a barbecue lighter) can #1.
- Measure the time that it takes for the flame to extinguish.
- When the flame goes out, note: did the powder/oil burn, or did you just burn off the rubbing alcohol? If the flame went out in less than about a second or two, you most likely just burned the alcohol. Also make note of the color of the fire and how it changes. Rubbing alcohol burns blue.
- You can add additional rubbing alcohol and attempt to light a 2nd or 3rd time if the powder/oil does not burn on the first attempt.
- Repeat steps 2-4 for each tin. Record your times.
If at any point a fire becomes uncontrolled, first try extinguishing it by covering the opening of the tin with a pot lid or ceramic plate. If this does not work, use your fire extinguisher or cover the fire completely with the fire blanket/damp towel. Do not pour water on grease fires (olive oil), as this will make them worse.
Activity 2: Cheesy Polymers
You’ve learned in this lesson that biomolecules are polymers, or long chains of the same repeated monomer. Another common polymer is plastic. Interestingly, a type of plastic polymer called casein can be made using the biomolecules found in cow’s milk!
Materials
1 cup of milk. Skim cow’s milk is preferred. The higher fat content in 2% or full-fat milk may change the consistency of your plastic, but it should still work okay. Plant-based milks will not make casein. You can use more if you want to make more casein, but be sure to increase the amount of vinegar proportionally.
4 tsp distilled white vinegar.
Microwave-safe cup/mug.
Paper towels. You need paper towels at a minimum. Higher absorbancies are preferred. You may also choose to use a cheesecloth or very fine strainer, in addition to paper towels.
Bowl or sink. For excess liquid.
Food coloring and/or cookie cutters (both are optional).
Check out this video for a tutorial:
4 tsp distilled white vinegar.
Microwave-safe cup/mug.
Paper towels. You need paper towels at a minimum. Higher absorbancies are preferred. You may also choose to use a cheesecloth or very fine strainer, in addition to paper towels.
Bowl or sink. For excess liquid.
Food coloring and/or cookie cutters (both are optional).
- Heat 1 cup of milk in a microwave until it is steaming, but not boiling. This should be about the same temperature as you would use for hot cocoa. This should take roughly 1-1.5 min in most microwaves.
- Add 4 tsp vinegar to the mug and mix well. You should notice curds forming.
- Stack a pile of 4-8 paper towels (depending on the absorbancy; too many is better than too few, but you can always add more) over a bowl or the sink.
- Pour the curdled milk on top of the paper towels or cheesecloth. The goal is to collect the curds but get rid of all of the liquid.
- Absorb any excess liquid still in the curds using the paper towels. You may want to collect the curds in a ball (inside of the paper towel/cheesecloth) and squeeze to get rid of excess liquid. You may need extra paper towels.
- Knead the curds together like you would with bread dough. This should reach the rougly the consistency of PlayDoh.
- You have just made casein plastic. Play around with it! You may choose to add food coloring. If you want, you can mold this into a shape and let it polymerize/dry over 48 hours.
Check out this video for a tutorial:
If you’re having fun or you making something you love, we want to see it! If you have an Instagram and the permission of your relevant responsible adult, share with us @eons_learning, #BioFireEons or #BiopolymerEons