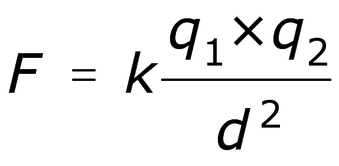Why Do Atoms Form Bonds?
Last lesson, we talked a lot about how important atoms are because they make up everything in the universe. Atoms are really important, and they are responsible for all of the important chemical reactions that keep interesting things happening all over the universe, from the furthest reaches of the farthest away galaxies down to your very core. But, now is the time to let you in on a little secret: Atoms can’t really do much all on their own. Sure, you have some cool elements like neon, that can make pretty colors all by themselves, and, sure, there are individual ions with very important functions, like sodium and potassium in your body, which are responsible for the processes that allow your brain and other nerve cells to work, but, for the most part, atoms need to make friends to do anything really cool. They need to get together. They need to unite for a larger purpose. Together, their potential to do awesome things is so much greater than it could ever be for just one single atom. When atoms get together to make friends, it is called chemical bonding.
So, how do atoms make friends? The simplest answer is with their electrons and, more specifically, with their valence electrons. Atoms “want” to be stable and have full valence shells, so they just share, trade, give away, and take electrons until everyone’s really happy and stable with all the electrons that they need. This takes some effort, to be sure: It can take quite a lot of energy to form a chemical bond. But, the stability is worth it. If you want to get a sense of how much energy is involved in chemical bonding, just look what happens when we do the opposite of forming a bond, and get back all that energy we put in by breaking a bond:
So, how do atoms make friends? The simplest answer is with their electrons and, more specifically, with their valence electrons. Atoms “want” to be stable and have full valence shells, so they just share, trade, give away, and take electrons until everyone’s really happy and stable with all the electrons that they need. This takes some effort, to be sure: It can take quite a lot of energy to form a chemical bond. But, the stability is worth it. If you want to get a sense of how much energy is involved in chemical bonding, just look what happens when we do the opposite of forming a bond, and get back all that energy we put in by breaking a bond:
That’s the energy of all those super-stable sugar molecules in those gummy bears being “torn” apart and turned into tiny carbon dioxide molecules. Boom.
So, chemical bonding involves super-important valence electrons, and it involves energy. But what exactly does all of this mean? How and why do bonds form?
Let’s take a deeper dive. There are two main ways to analyze a chemical bond: from the perspective of physics or from the perspective of chemistry. Both ways contribute greatly to our understanding of how bonds work.
Electrostatic forces: the physics persepective
Just about any physicist will tell you that bonds happen because of electrostatic forces. Opposites attract; positives attract negatives. With this little fact, you can really explain a whole lot of what goes on in the universe.
Electrostatic forces (interactions between charges) cause the electrons of one atom to be attracted to the protons of the other atom. Electrostatic forces are explained by Coulomb’s Law:
Electrostatic forces (interactions between charges) cause the electrons of one atom to be attracted to the protons of the other atom. Electrostatic forces are explained by Coulomb’s Law:
Where F is the force (amount of attraction) between two charges (q1 and q2), q is the strength of the charge (1, 2, 3, etc.), and r is the distance between the two charges.
For those of you who aren't yet fluent in math, this equation tells us two things:
And in even more direct terms:
When both of these things happen, positive attracts negative, and two atoms are held together in harmony.
For those of you who aren't yet fluent in math, this equation tells us two things:
- Charges close together will form stronger bonds. Charges farther apart will form weaker bonds.
- Bigger charges will form stronger bonds. Smaller charges will form weaker bonds.
And in even more direct terms:
- Atoms have to be close together to form a bond. In fact, they have to collide (bump together). The more “crowded” a given space is with atoms, the more likely it is that chemical bonding will occur.
- Atoms have to be charged to form a bond. This can either be a complete charge (as is the case with ionic bonds), or a partial charge (as is the case with covalent bonding and most forms of intermolecular attraction). We’ll learn about these specific types of bonding later. For now, focus on: The bigger the charge, the stronger the bond.
When both of these things happen, positive attracts negative, and two atoms are held together in harmony.
Thermodynamics: The chemistry perspective
Perfect, right? That explains everything.
Well, not quite. Although it is true that positives attract negatives, not every positive attracts every negative in quite the same way. Sometimes, the positive will attract two negatives, and sometimes only one. Sometimes, certain positives won't attract certain negatives very well, if at all. To understand this, we have to turn to the chemist.
"Hey, chemist, how's it going?"
"Oh, gee, intrigued student of science, I'm doing just spectacularly, and yourself?"
"Well, I tell you, chemist, I'm baffled. I just can't seem to get these two atoms to fit together the way that I want. You see, I want to stick two of these negatives onto this here positive, but the positive will only take one!"
"You make an interesting point, intrigued student of science. Let me teach you a little bit about thermodynamics."
Put simply, thermodynamics is the study of stability. As you’ve quite recently learned, stability is basically just how "happy" an atom (or anything) is. If it is high in energy, it will be less stable. Energy has to come from somewhere, and there just isn't always enough of it to fuel a high-energy state. Atoms (and everything) want to be low in energy. Why? Because it's easier that way. Things are lazy. (This explanation is, of course, not totally scientific, but it works).
As it happens, atoms are low in energy (stable) when their valence shells are full. Remember what a valence shell is?
Well, not quite. Although it is true that positives attract negatives, not every positive attracts every negative in quite the same way. Sometimes, the positive will attract two negatives, and sometimes only one. Sometimes, certain positives won't attract certain negatives very well, if at all. To understand this, we have to turn to the chemist.
"Hey, chemist, how's it going?"
"Oh, gee, intrigued student of science, I'm doing just spectacularly, and yourself?"
"Well, I tell you, chemist, I'm baffled. I just can't seem to get these two atoms to fit together the way that I want. You see, I want to stick two of these negatives onto this here positive, but the positive will only take one!"
"You make an interesting point, intrigued student of science. Let me teach you a little bit about thermodynamics."
Put simply, thermodynamics is the study of stability. As you’ve quite recently learned, stability is basically just how "happy" an atom (or anything) is. If it is high in energy, it will be less stable. Energy has to come from somewhere, and there just isn't always enough of it to fuel a high-energy state. Atoms (and everything) want to be low in energy. Why? Because it's easier that way. Things are lazy. (This explanation is, of course, not totally scientific, but it works).
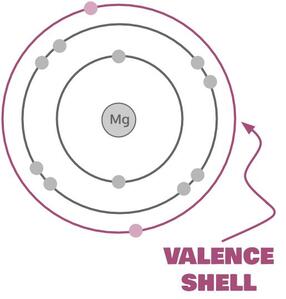
As it happens, atoms are low in energy (stable) when their valence shells are full. Remember what a valence shell is?
That's a valence shell. It's an atom’s outer shell of electrons in a Bohr model. As you learned when we talked about Bohr models, valence shells tend to be full when they have 8 electrons (except hydrogen and helium, which are full with 2, and stuff way down on the periodic table, where stuff gets wonky). This is called the octet rule. It's really more of a guideline than a rule, but it's still quite handy.
The octet rule is one of many attempts to explain chemical bonding using thermodynamics. The rest are a bit above your pay grade, but they all boil down to the same basic point: Atoms want to be stable. Forming a bond with something requires energy, so the bond won't form if it doesn't get back at least that much energy in return. Why? Because that would make it less stable. A stable atom is a happy atom.
So, how can you use this knowledge in terms of chemical bonding? Read on, my friend.
The octet rule is one of many attempts to explain chemical bonding using thermodynamics. The rest are a bit above your pay grade, but they all boil down to the same basic point: Atoms want to be stable. Forming a bond with something requires energy, so the bond won't form if it doesn't get back at least that much energy in return. Why? Because that would make it less stable. A stable atom is a happy atom.
So, how can you use this knowledge in terms of chemical bonding? Read on, my friend.
Summary
You should understand:
- That atoms must collide (bump together) in order to form chemical bonds.
- That chemical bonding involves atoms being held together by electrostatic forces (positive charges attracting negative charges), which become stronger as the magnitudes of these charges become stronger.
- That atoms will continue to react until they become most stable.
- How the concept of stability, the octet rule, and the number of valence electrons relate to one another.
Learning Activity
Content contributors: Emma Moulton, Eli Levine, Emily Zhang