Modeling Covalent Molecules
In the previous lesson, you learned how to draw Lewis Dot Structures to show how cations give and anions receive an electron to complete their valence shells, and in that love, and the having and giving and sharing and receiving, we too can learn to have, and give, and share, and receive. In this lesson, you’ll learn how to do the same for the less give-and-take and more sharing-is-caring covalent compounds, which share a pair of electrons between two shells.
Lewis dot structures are the picture version of covalent molecules. A covalent bond is shown with a line, and leftover or nonbonded electrons are shown by a pair of dots. For all the molecules we’ll have you draw in this class, you will never ever end up with a single dot (a single nonbonded electron)--they will always come in pairs. There are exceptions to this rule, one of which we saw earlier with the air pollutant NO₂, but we won’t worry about them.
To draw lewis dot structures, follow these three simple (possibly, kind of complicated) steps:
- Draw the Lewis Dot structures of each atom in the molecule. You should remember these from when we made models for ionic bonding.
- “Share Pair” the electrons in each structure (Circle 2 electrons, connecting two atoms) to indicate that two atoms “share” a pair of electrons. The ultimate goal is for each atom to have or share 8 electrons, or 2 electrons for hydrogen, giving them a stable noble gas configuration. Up to 3 pairs can be formed between two atoms; this represents a double bond or triple bond. Only use as many bonds as you need to give every atom an octet!
- Replace each pair of bonded electrons with a straight line. This represents a covalent bond. For double/triple bonds, use two or three parallel lines. Leave nonbonded pairs as dots. Make sure every dot has a pair! If it doesn't, you did it wrong.
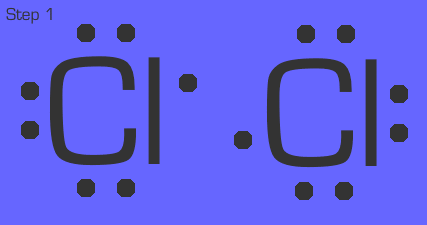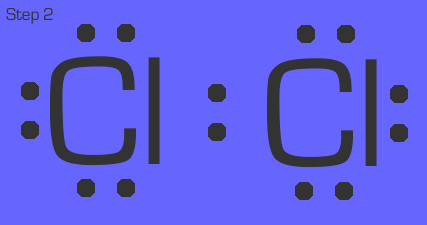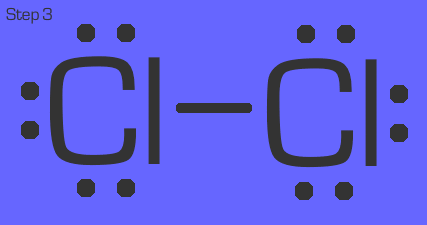
Chlorine is a quick example. Each chlorine in Cl₂ (remember, this is a diatomic gas) has 1 nonbonded electron. They simply share that electron so that they both have an octet.
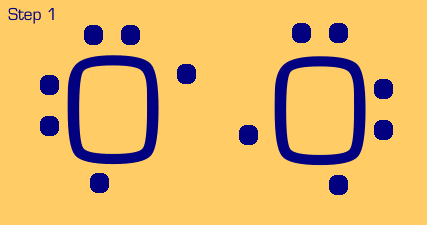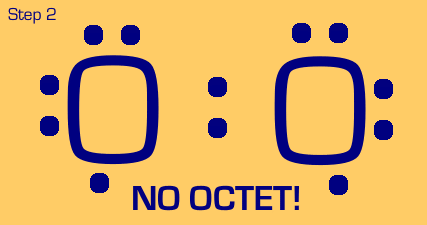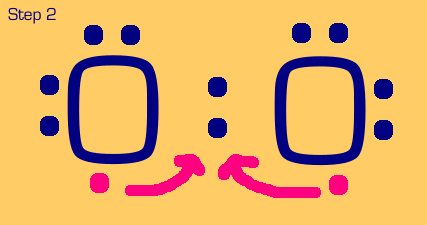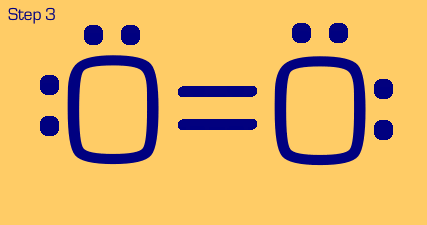
Oxygen is a somewhat tougher example. We see that when we move two electrons to the center to make a bond, each oxygen still only has (or thinks it has) 7 electrons, when really it wants eight. We also see that we have two nonbonded electrons that are not part of a lone pair. These are no-nos. So, we have to “share pair” again until we get a full octet on each, and no unpaired nonbonded electrons.
If we were to “share pair” again and we still didn’t have a full octet on each and/or we still had unpaired nonbonded electrons, we would have to “share pair” yet again to make it work. We see this in nitrogen (N₂, another diatomic gas):
If you ever end up with more than a full octet, at least for the examples you will see in this class (down farther on the Periodic Table we can sometimes see something called an expanded octet, but don’t worry about this for now), you’ll know you’ve made a mistake. If you can’t find that mistake easily, you’ll have to go back to the drawing board. It’s like solving a puzzle.
A Rule of Thumb for Covalent Bonding
Here's a little shortcut to what you just learned: for the molecules you will see in this class, the number of bonds an atom needs to form to make an octet is the same number of spaces it is from the last column of the periodic table. For example:
Group |
Elements Included |
Bonds Formed |
Example With Structure |
14 |
C (sometimes Si) |
4 |
CO₂ --> O=C=O |
15 |
N, P |
3 |
N₂ --> N≡N |
16 |
O, S |
2 |
O₂ --> O=O |
17 (aka halogens) |
F, Cl, Br, I |
1 |
Cl₂ --> Cl-Cl |
hydrogen |
H |
1 |
H₂ --> H-H |
This is kind of like doing a little sneak preview of what the puzzle “should” look like when it’s done—and will hopefully help you to puzzle things together a little more quickly.
Summary
You should know:
As always, the more you practice with this, the easier it gets.
We will soon be leaving chemical bonding and reactions for a while to study the properties of matter. But, have no fear, your friend the molecule is still going to be with us to explain many of the properties of all ordinary matter.
- How to draw Lewis dot structures for covalently bonded molecules, with single bonds, double bonds, and triple bonds.
- How to tell if an atom has a noble gas configuration (octet) using Lewis structures.
As always, the more you practice with this, the easier it gets.
We will soon be leaving chemical bonding and reactions for a while to study the properties of matter. But, have no fear, your friend the molecule is still going to be with us to explain many of the properties of all ordinary matter.
Learning Activity
| LA Modeling Covalent Molecules |
This is another one of those things that you have to do in order to learn. After all, would you ever solve a jigsaw puzzle just by having someone tell you the basic rules of how to put together a jigsaw puzzle? Of course not! And this is just like that—I’ve given you the rules and some basic instructions, but now it’s your turn to actually learn how to draw molecular models.
For the following, you may draw out your answers in any format you like, but it is probably easiest to do them on a piece of paper.
For the following, draw the appropriate molecular structure. Some are standard nomenclature, and some are names that I had previously asked you to be familiar with; you may wish to review the associated nomenclature lesson. Be sure to include all lone pairs in your structures.
Challenge problem: Draw ethanol (C₂H₅OH) and briefly state where you might find it. Be sure to follow the drawing conventions we use in this class! (The same ones you have been using all lesson).
For the following, you may draw out your answers in any format you like, but it is probably easiest to do them on a piece of paper.
For the following, draw the appropriate molecular structure. Some are standard nomenclature, and some are names that I had previously asked you to be familiar with; you may wish to review the associated nomenclature lesson. Be sure to include all lone pairs in your structures.
- Carbon dioxide
- Nitrogen (Remember HOFBrINCl!)
- Boron trifluoride (Hint: Boron is an exception. It’s “octet” is full with 6 electrons.)
- Methane
- Ammonia
- Water
- Sulfur hexafluoride (Hint: Sulfur can have an expanded octet. It will have 12 electrons in its valence shell in this structure.)
Challenge problem: Draw ethanol (C₂H₅OH) and briefly state where you might find it. Be sure to follow the drawing conventions we use in this class! (The same ones you have been using all lesson).
Content contributors: Eli Levine, Nancy Jiang, Emma Moulton