Glycolysis and Anaerobic Respiration
Making ATP
We’ve talked a lot about a molecule called ATP. ATP is the molecule that makes it possible to do all kinds of difficult, crazy reactions in the cell. It makes reactions that wouldn’t normally want to happen happen really easily, by providing an energy incentive. In the simplest possible terms, you can think of ATP like a biochemical deus ex machina that just makes life happen by throwing in some hand-wavy, magicky energy. Like Doctor Who’s sonic screwdriver. Or Gandalf.
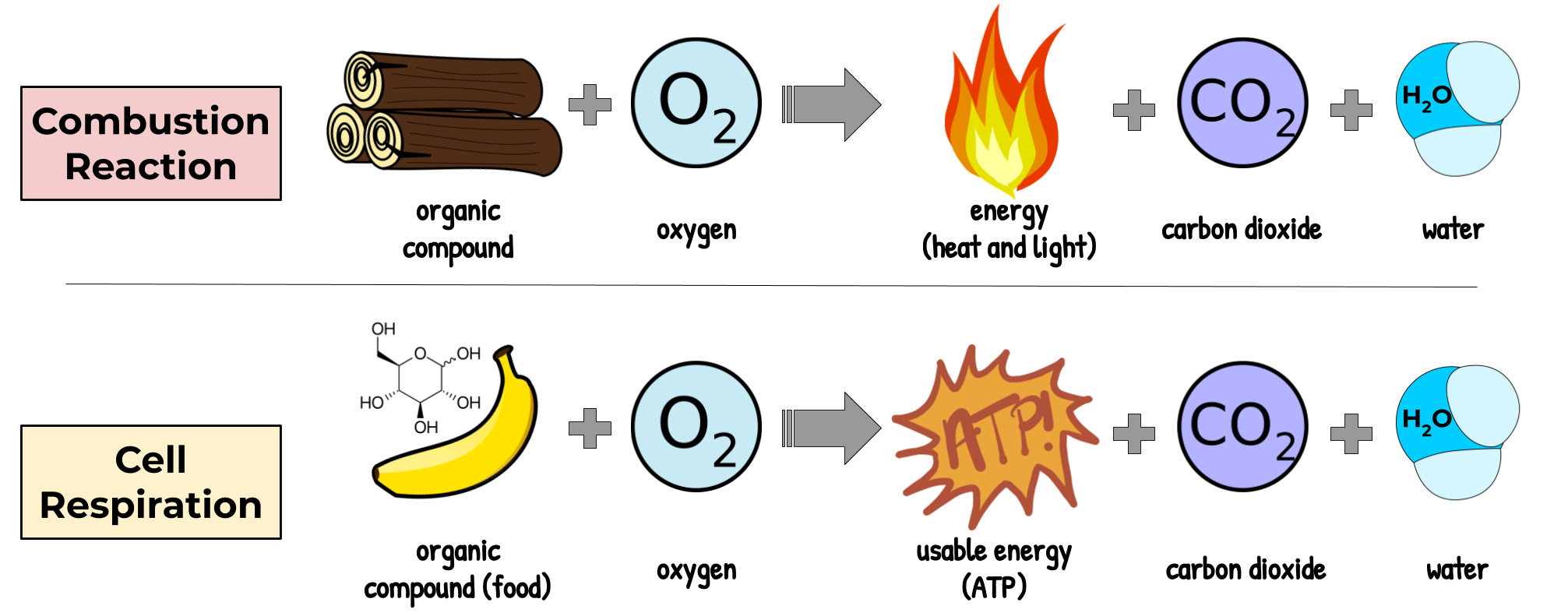
Because ATP’s magic third phosphate makes the molecule very unstable (which is how it can power chemical reactions), ATP must be constantly produced by the cell. It can’t be stored. This happens through a process called cellular respiration. The main goal of this process is to get the energy out of sugar. But, instead of just letting this energy escape in the form of heat and light, like in a standard combustion reaction, we have to break down glucose one step at a time and collect the energy as we go, in a way that can be used to make ATP.
Let’s take a closer look at how our cells very carefully “explode” sugar.
Glycolysis
The first step of cellular respiration (of glucose) is glycolysis. Glycolysis literally means “breaking apart sugar” (glyco = “sugar,” lysis = “breaking apart”).
Glycolysis takes glucose, which is made up of 6 carbons, and breaks it into 2 pyruvate molecules, which are 3 carbons each.
Overall, energy is released when you turn glucose into pyruvate. Since the main goal is to capture that energy, that must mean some new energy-storing molecules were made. In this case, the energy “reward” for turning glucose into 2 pyruvate is 2 ATP plus 2 NADH. You don’t need to memorize these numbers for this class.
The ATP that is made by glycolysis can immediately be used to power chemical reactions in the cell. NADH is also a high-energy molecule, but it isn’t used for energy right away. Instead, it holds onto that energy for now and can be used to make a lot more ATP later on in organisms that can do aerobic respiration, which is the type of respiration that uses oxygen (we do this!). We’ll continue our learning of cell respiration by going through aerobic respiration in more detail in the next part of this lesson.
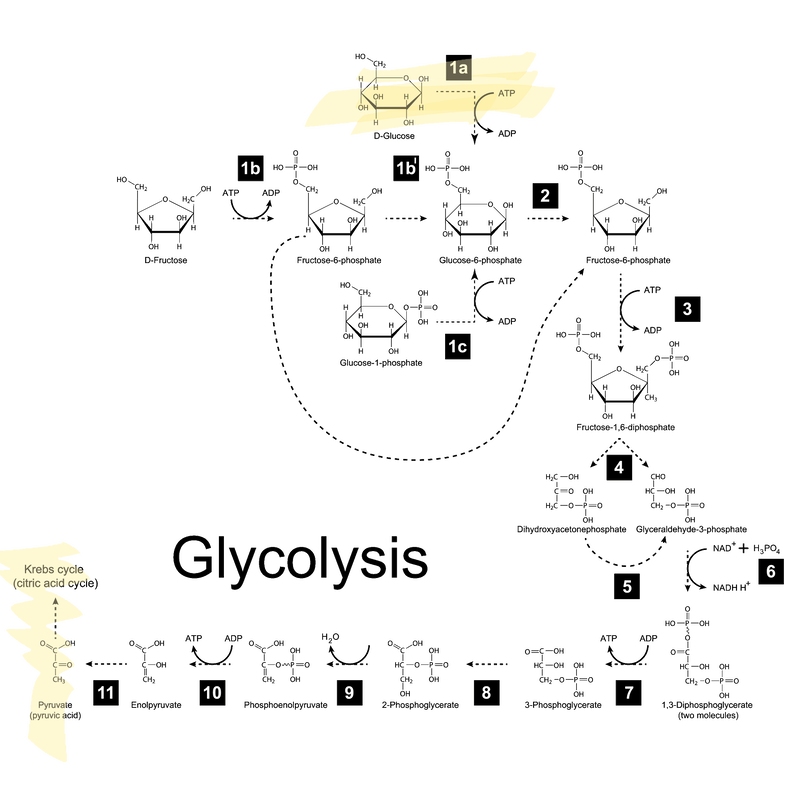
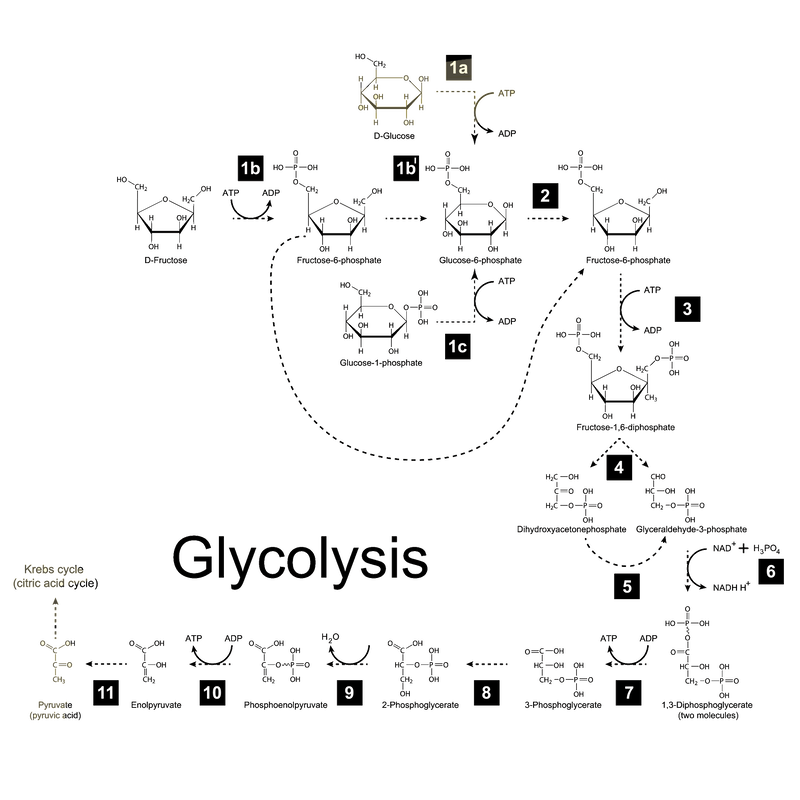
There is an actual chemical reaction that explains just how ATP and NADH are made by breaking apart glucose--but you don’t have to worry about that for this class. It’s much more important for you to understand the main idea that we are gradually breaking down glucose, releasing energy along the way and storing that energy in ATP and NADH. If you just want to be convinced that cells do chemistry and not magic, here’s a picture of what the actual mechanism looks like:
Glycolysis takes glucose, which is made up of 6 carbons, and breaks it into 2 pyruvate molecules, which are 3 carbons each.
Overall, energy is released when you turn glucose into pyruvate. Since the main goal is to capture that energy, that must mean some new energy-storing molecules were made. In this case, the energy “reward” for turning glucose into 2 pyruvate is 2 ATP plus 2 NADH. You don’t need to memorize these numbers for this class.
The ATP that is made by glycolysis can immediately be used to power chemical reactions in the cell. NADH is also a high-energy molecule, but it isn’t used for energy right away. Instead, it holds onto that energy for now and can be used to make a lot more ATP later on in organisms that can do aerobic respiration, which is the type of respiration that uses oxygen (we do this!). We’ll continue our learning of cell respiration by going through aerobic respiration in more detail in the next part of this lesson.
There is an actual chemical reaction that explains just how ATP and NADH are made by breaking apart glucose--but you don’t have to worry about that for this class. It’s much more important for you to understand the main idea that we are gradually breaking down glucose, releasing energy along the way and storing that energy in ATP and NADH. If you just want to be convinced that cells do chemistry and not magic, here’s a picture of what the actual mechanism looks like:
If it’s helpful for your conceptual understanding to see how these steps fit together, you can watch this video. We’re mainly interested in you understanding the main idea of glycolysis, including the start and end products, than the process:
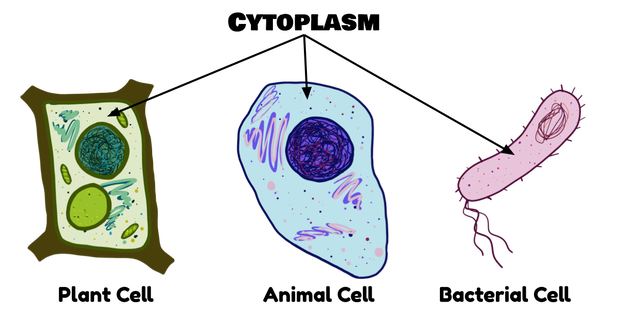
Glycolysis occurs in all living organisms (including bacteria) and does not require oxygen. All the other steps of aerobic respiration don’t happen in most types of bacteria, but do happen in all eukaryotes (plant and animal cells), and don’t happen unless oxygen is present.
This process happens in the main compartment of the cell, which is called the cytoplasm. The cytoplasm is all the goopy, gooey stuff that is inside of the cell membrane but isn’t inside of an organelle.
Anaerobic Respiration
After glycolysis, most eukaryotic cells continue to break down pyruvate from cellular respiration and release all the energy from it. This is called aerobic respiration, and it requires oxygen and specialized machinery found in organelles called mitochondria. In these cells, cell respiration starts with glycolysis and continues through both steps of aerobic respiration. For now, we mainly want you to focus on understanding glycolysis, which is the first step in this process and happens in all living things.
Living things that can’t do aerobic respiration, whether because they lack the machinery (as is the case with many bacteria) or because they don’t have enough oxygen (as is the case with, for example, our muscle cells when they’re working really hard), get all of their energy from glycolysis. This means they have to take up a lot of sugar to get proportionally less energy. It’s not that efficient, but it will do in a pinch. This is called anaerobic respiration, or respiration that does not require oxygen (“an-” as a prefix means “not”).
But, glycolysis can’t just keep going on forever, because you’ll run out of the important, difficult-to-make-or-find molecule NAD⁺. NAD⁺ is the resource that gets turned into NADH. In aerobic respiration, we’ll never run out of NAD⁺ because, once NADH is used in the electron transport chain, we get our NAD⁺ back. In anaerobic respiration, we need a second step to recycle NAD⁺. This can happen one of two ways:
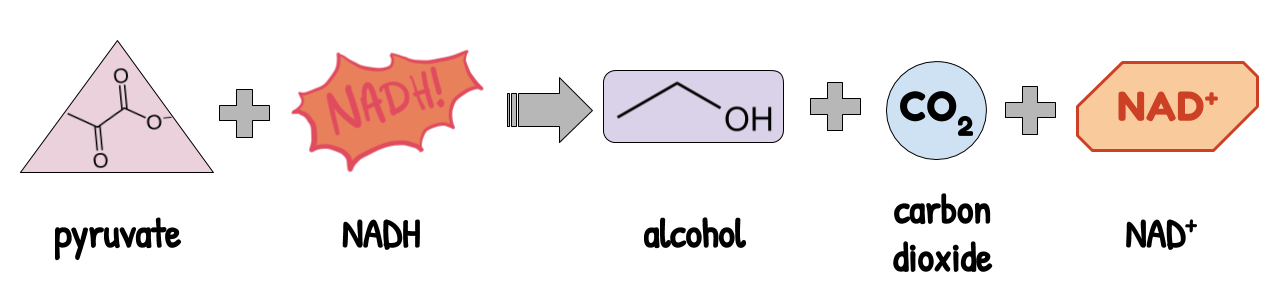
1. Alcoholic fermentation is done by yeast. It is how beer and bread is made. It recycles NADH by turning pyruvate (3 carbons) into CO₂ (1 carbon) and alcohol (2 carbons).
Living things that can’t do aerobic respiration, whether because they lack the machinery (as is the case with many bacteria) or because they don’t have enough oxygen (as is the case with, for example, our muscle cells when they’re working really hard), get all of their energy from glycolysis. This means they have to take up a lot of sugar to get proportionally less energy. It’s not that efficient, but it will do in a pinch. This is called anaerobic respiration, or respiration that does not require oxygen (“an-” as a prefix means “not”).
But, glycolysis can’t just keep going on forever, because you’ll run out of the important, difficult-to-make-or-find molecule NAD⁺. NAD⁺ is the resource that gets turned into NADH. In aerobic respiration, we’ll never run out of NAD⁺ because, once NADH is used in the electron transport chain, we get our NAD⁺ back. In anaerobic respiration, we need a second step to recycle NAD⁺. This can happen one of two ways:
1. Alcoholic fermentation is done by yeast. It is how beer and bread is made. It recycles NADH by turning pyruvate (3 carbons) into CO₂ (1 carbon) and alcohol (2 carbons).
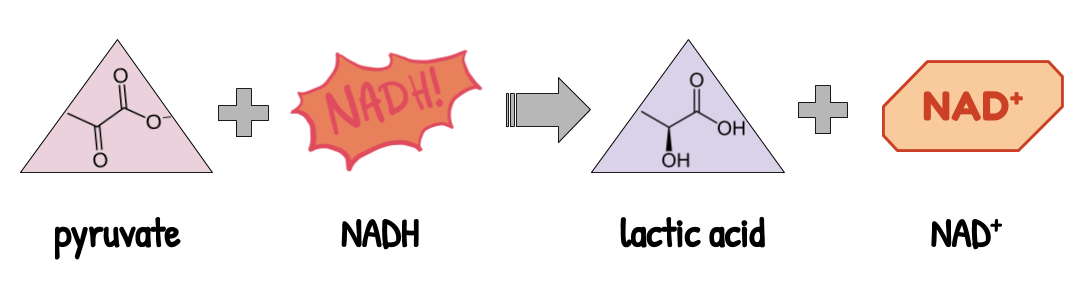
2. Lactic acid fermentation is done by muscle cells during exercise. This type of fermentation is also present in the bacteria found in yogurt, which convert lactose into lactic acid. It recycles NADH by turning pyruvate (3 carbons) into lactic acid (3 carbons). No CO₂ is produced, since both pyruvate and lactic acid have 3 carbons.
Even though anaerobic respiration is inefficient, nearly all organisms show some form of anaerobic fermentation, indicating that this process likely occurred in early evolutionary history--perhaps in places where oxygen was hard to come by, like deep in the ocean or in thermal vents. And, even though it’s inefficient, anaerobic respiration is still better than no respiration at all—and it’s definitely great for fans of yogurt and bread.
This video gives a good overview of anaerobic respiration:
This video gives a good overview of anaerobic respiration:
Summary
You should understand:
- That cell respiration is the process of gradually breaking down glucose and collecting usable energy from it.
- That glycolysis is the first step of aerobic respiration and is the only energy-producing step in anaerobic respiration.
- That glycolysis breaks down glucose (6 carbons) into 2, 3-carbon pyruvate molecules, and that the energy “reward” for this is ATP and NADH. It happens in the cytoplasm.
- That aerobic respiration is the preferred way of making energy, when it is possible, because it is more efficient (you get more ATP per glucose—by a lot).
- That NAD⁺ must be recycled in anaerobic respiration, which happens through either alcoholic fermentation or lactic acid fermentation.
Learning Activity
Content contributors: Kathleen Yu, Eli Levine, Emma Moulton