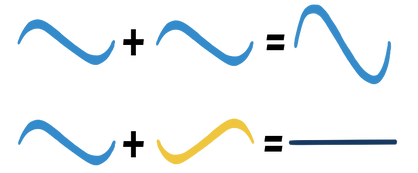Covalent Bonding
Sharing is Caring
As a refresher, in order for atoms to bond with each other, they need to either donate or share valence electrons to attain a stable configuration (like an octet). Donating electrons forms an ionic bond; sharing electrons forms a covalent bond. When atoms don’t bond with each other to attain a more stable configuration, they are sad. When atoms do bond with each other to attain a more stable configuration, they are happy!
As you learned, an ionic bond will only form between a metal and a nonmetal. In the same token, a covalent bond will only form between two nonmetals.
Most nonmetals have a lot of electrons in their valence shells, so it takes A LOT of energy to make one of them a cation. Chlorine (Cl), for example, would need to lose seven electrons to look like neon (Ne), whereas it only needs one more to look like argon (Ar), and it’s happy as either noble gas. Since you need a cation for an ionic bond, it just doesn’t happen with two nonmetals: it would take way to much energy to give up enough electrons for one of the nonmetals to become a cation. So, instead of completely selling off their electrons, our nonmetal friends just share with each other, and everybody wins. Sharing is caring!
Most nonmetals have a lot of electrons in their valence shells, so it takes A LOT of energy to make one of them a cation. Chlorine (Cl), for example, would need to lose seven electrons to look like neon (Ne), whereas it only needs one more to look like argon (Ar), and it’s happy as either noble gas. Since you need a cation for an ionic bond, it just doesn’t happen with two nonmetals: it would take way to much energy to give up enough electrons for one of the nonmetals to become a cation. So, instead of completely selling off their electrons, our nonmetal friends just share with each other, and everybody wins. Sharing is caring!
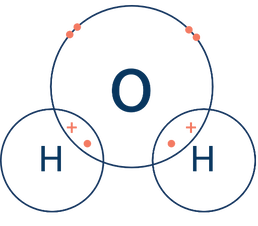
Just like NaCl is a good model for ionic bonding, water has become the poster child of covalent bonds. A water molecule, or H₂O, looks like this:
In this picture, you can see that oxygen thinks it has 8 electrons in its valence shell (a stable octet), but it actually only owns 6 of these electrons. Likewise, hydrogen is super happy with its 2 electrons, but really it only owns 1. It’s kind of like a timeshare for electrons.
(A little tip on the octet rule, which says that atoms want 8 electrons: since it's more of a guideline, it may be more useful to remember that noble gases are perfectly stable. Atoms will bond until they look like noble gases.)
The real trick with covalent bonding is trying to figure out an arrangement that makes everybody happy. Atoms, of course, don't even have to think about it. They will automatically go to the most stable configuration—the best way of sharing—when they come in contact with another atom. But, for us mere mortals, we have to solve the puzzle. You'll have a lot of practice with this when we talk about molecular models. For now, our real question is: Why do covalent bonds form? What holds them together?
Why Do Covalent Bonds Form?
Much of this question can be answered with electrostatic attraction and stability, which you learned when we talked about the basics of chemical bonding. With covalent bonds, though, it goes a bit further. A lot of people have trouble wrapping their heads around the concept that I'm about to explain, so don't freak out if you're one of these people. It is just to show you that the universe makes sense, and to illustrate the point that science doesn't just happen because scientists said so. It all comes back to the fundamental laws of the universe. As long as you understand this point, you're in good shape. In other words, put down your pens and stop taking notes, but do keep reading.
Remember when I said that electrons are sort of like particles and sort of like waves? Well, they're actually mostly like waves. Yes, like a wave in the ocean, or like a sound wave, or like a heat wave, or like a wave of light. (Mostly. They're 3-D, and these other kinds of waves aren't. But the concept still stands. Hehe. Get it? Stands. Oh, wait, I haven’t told you that they’re called standing waves yet. Inside joke, inside joke.)
Electron waves can add together in one of two ways: to make one big wave, or to make no wave at all.
Remember when I said that electrons are sort of like particles and sort of like waves? Well, they're actually mostly like waves. Yes, like a wave in the ocean, or like a sound wave, or like a heat wave, or like a wave of light. (Mostly. They're 3-D, and these other kinds of waves aren't. But the concept still stands. Hehe. Get it? Stands. Oh, wait, I haven’t told you that they’re called standing waves yet. Inside joke, inside joke.)
Electron waves can add together in one of two ways: to make one big wave, or to make no wave at all.
This is called interference. It is a property of all waves.
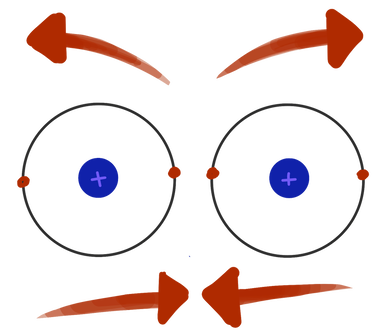
So, when electron waves get close together, they can make one big wave—which is a bond—or no wave at all—an anti-bond. As it turns out, it takes less energy to make a bond, because the nuclei are now both attracted to the electrons instead of repelling each other (the electrons shield them from the high-energy, repulsive positive charges). Hydrogen gas (H₂) is the simplest example of this, because only 2 electrons are brought in, which is exactly how many you need for a bond.
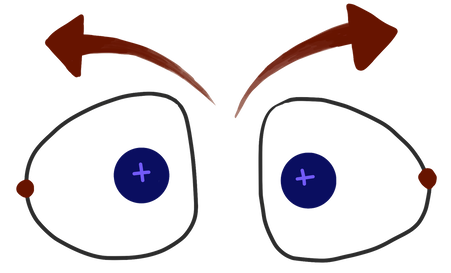
Hydrogen gas anti-bond
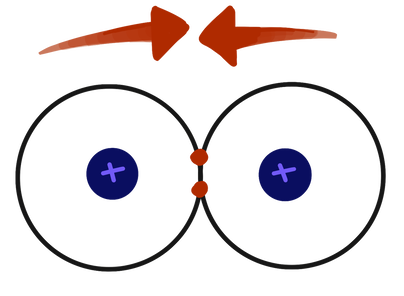
Hydrogen gas bond
But, we know that helium doesn’t form He₂: it stays all by itself. Why?
Well, atoms will form a bond if they can—but, an atom can only create a certain number of bonds. After that, its only choice is to make anti-bonds. It’s like how you would rather buy things than sell them, but at some point you’ll run out of money if you only ever buy things. If too many electrons are brought into the picture, they will form an anti-bond, even though it would be easier to make a bond. Eventually, the number of anti-bonds cancels out the number of bonds, and you get no bonds. And guess what? This number of bonds and anti-bonds that atoms can form goes right along with what you've already learned about the octet rule.
Bond anti-bond
Okay, that's enough quantum physics. Cool, no?
Polarity: Sharing is Not Always Equal
Now that you’ve got the basics (and some of the advanced stuff) about why bonds form, it’s time to add one little qualifier: Not all bonds involve equal sharing. This idea is really important when we talk about the stuff that molecules do, like bond with each other (yep, molecules bond, too) in intermolecular bonds, and react with each other in chemical reactions.
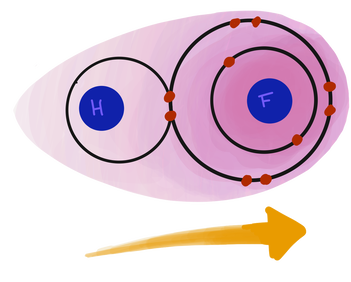
Polarity is the unequal distribution of negative charge in a molecule because of unequal sharing of electrons. This happens because not all elements like sharing as much as others. Elements that don’t like to share are considered electronegative, or electron hogs. (They are negative because they hoard all of the electron density, making them negatively charged. And because that’s mean, making them “negative” like “bad.”) Electronegativity basically describes how much an element “wants” electrons (or how much is doesn’t want to share).
Sometimes, one atom attracts more strongly and pulls the electrons to one end of the bond, creating an area of negative charge around that atom (remember: Electrons are negative). As a result, the now “bare” atom on the other end of the bond has a (partial) positive charge. It’s like tug-of-war, and the more electronegative element always wins. A bond is termed polar if this “tug-of-war” occurs.
Polarity is the unequal distribution of negative charge in a molecule because of unequal sharing of electrons. This happens because not all elements like sharing as much as others. Elements that don’t like to share are considered electronegative, or electron hogs. (They are negative because they hoard all of the electron density, making them negatively charged. And because that’s mean, making them “negative” like “bad.”) Electronegativity basically describes how much an element “wants” electrons (or how much is doesn’t want to share).
Sometimes, one atom attracts more strongly and pulls the electrons to one end of the bond, creating an area of negative charge around that atom (remember: Electrons are negative). As a result, the now “bare” atom on the other end of the bond has a (partial) positive charge. It’s like tug-of-war, and the more electronegative element always wins. A bond is termed polar if this “tug-of-war” occurs.
Polar Bond
This pulls electron density away from the bond, which makes that bond weaker and more likely to break in a chemical reaction. It also puts a partial positive charge on one side of the molecule and a partial negative charge on the other side of the molecule, which allows electrostatic interactions to occur.
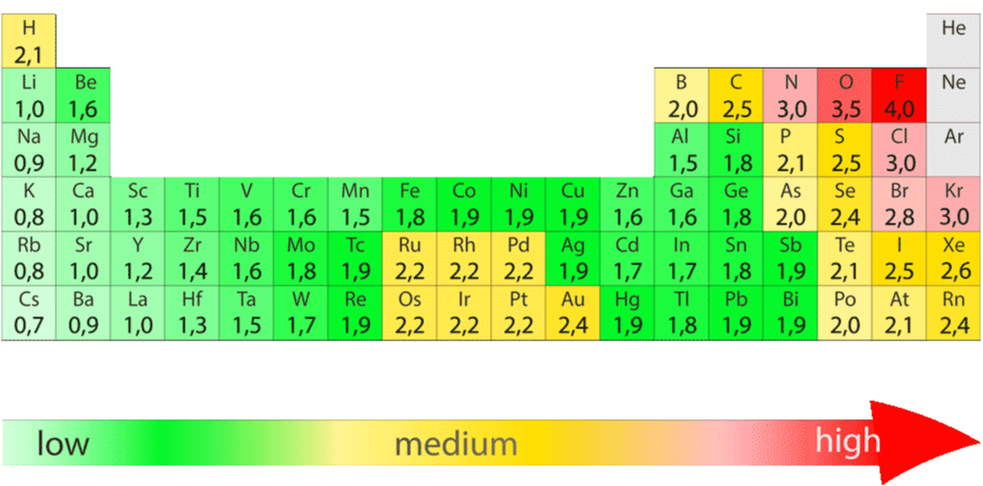
Electronegativity is measured on a relative scale, with fluorine being the highest at 4.0. Other electronegativities are provided below. The closer you get to fluorine on the Periodic Table, the more electronegative you are.
ELECTRONEGATIVITY
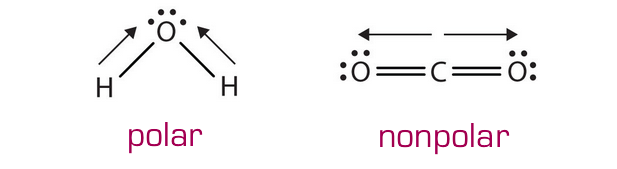
The polarity of a molecule depends on both the polarity of its bonds and the shape of the molecule as a whole: That is, if one element if pulling electrons towards one end of the molecule, it will be polar, but if electrons are being pulled in opposite directions, the effect will cancel out, as shown below.
This video provides a great overview of polarity:
Summary
You should understand:
This video provides a great overview of these points, as well as other topics we’ve addressed so far in chemical bonding.
- That atoms share electrons in a covalent bond.
- That covalent bonds form between two nonmetals.
- That all of the atoms you will be working with (except hydrogen and helium) follow the octet rule. This means that they "want" to have 8 electrons in their valence shell. Hydrogen and helium want 2. (The always-true rule is that atoms “want” to look like noble gases).
- That any bonded molecule that (semi-permanently) stays a bonded molecule is more stable than the atoms would be separately. Otherwise, they wouldn't bother to form a bond.
- That electronegative elements will pull electron density toward them and out of a bond, making that portion of the molecule relatively more negative.
This video provides a great overview of these points, as well as other topics we’ve addressed so far in chemical bonding.
Understand these points, and you will be well prepared to understand the other chemistry we will deal with in this course. Covalent bonding is a very important concept in molecular chemistry. If any of the things in this recap were news to you, it may be worth going back and taking a second look at this and past lessons.
Learning Activity
Content contributors: Emma Moulton, Eli Levine, Emily Zhang