The Structure of the Cell Membrane
In the last part of this lesson, you learned that cell membranes are really special and important because they make sure that everything that’s inside the cell is really special and important:
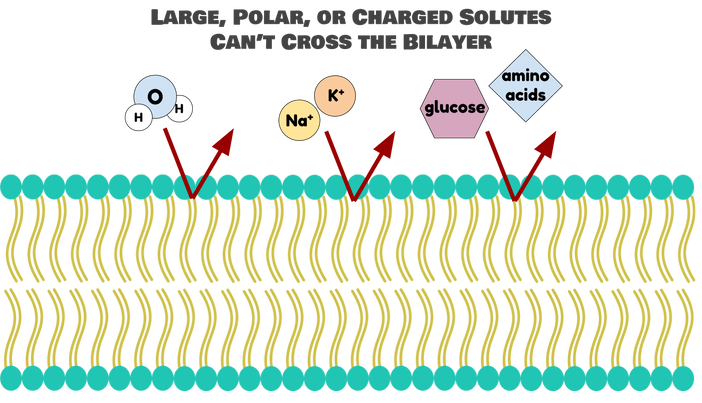
They act like the bouncer at a swanky club, letting in only specific molecules:
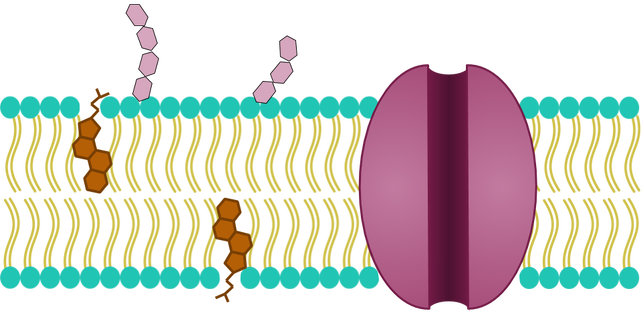
Before we get into the precise how of how the cell membrane keeps the inside of the cell so special, we first need to talk about what the cell membrane is made of and how each of these individual components contribute to the overall goal of the cell membrane. As a reminder, the cell membrane looks a little something like this:
There are 3 main elements you should see here: lipids, proteins, and carbohydrates. You learned about the general functions of these macromolecules in the biochemistry lesson, but now we’ll talk about the specific functions that pertain to the cell membrane.
Lipids
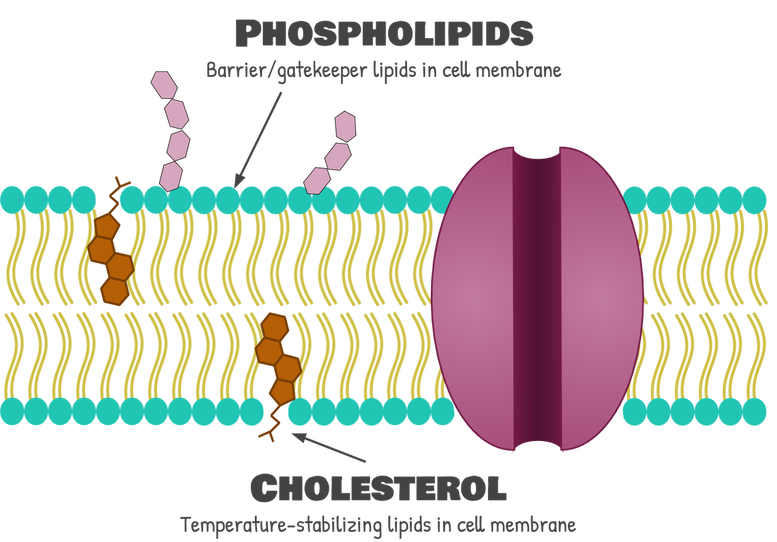
By far the most abundant element of the cell membrane is the lipids. More specifically, the cell membrane is mostly made up of a type of lipid called phospholipids. In fact, the cell membrane is sometimes referred to as the phospholipid bilayer because it is 2 layers of phospholipids (we don’t like this term as much to describe the whole cell membrane, because there’s a lot of other stuff going on in the cell membrane, too). There is also some cholesterol in there, which, as you may remember, is important because it keeps the membrane fluid.
Phospholipids (more specifically the phospholipid tails) make up the actual “fence” part of the membrane: They are a physical barrier that blocks everything but small, nonpolar molecules from getting in (more on this in a minute). Phospholipids have a special function, so they must also have a special structure:
Phospholipids (more specifically the phospholipid tails) make up the actual “fence” part of the membrane: They are a physical barrier that blocks everything but small, nonpolar molecules from getting in (more on this in a minute). Phospholipids have a special function, so they must also have a special structure:
|
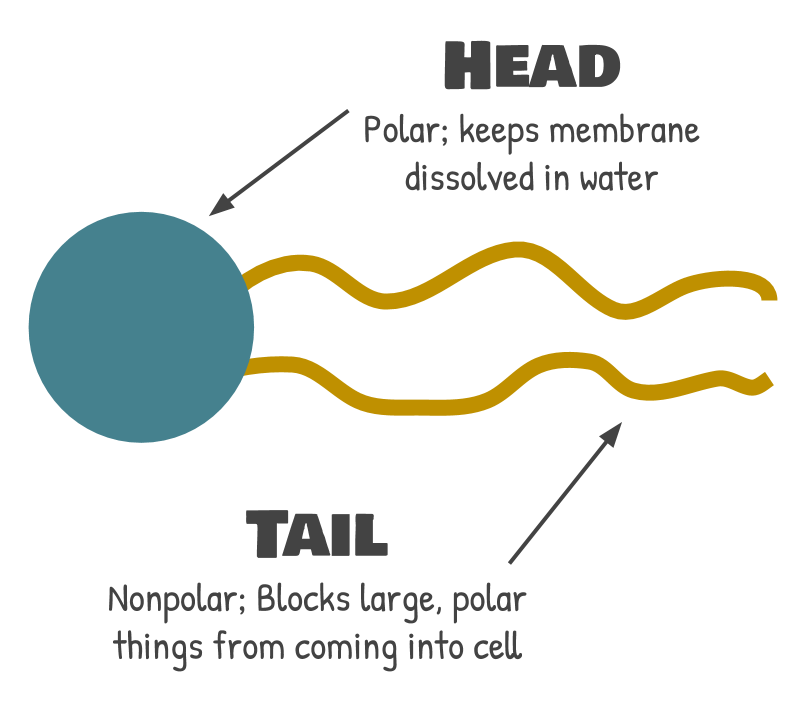
Phospholipids have a phosphate group in the head and a two fatty acid chains (the monomers of lipids) in the tail. This is why they are called “phospho” (for phosphate) “lipids” (for the fatty acids).
Phosphate groups are very polar, or hydrophilic: they love hanging around with water (“hydro” means water and “philic” means loves: so hydrophilic things love water). This keeps the membrane dissolved in water: the head is able to dissolve in water, while the oily part can hide away inside of the bilayer and never have to touch water. Because the polar head will always flip to dissolve in water and the tail will always flip to hang out with other tails instead of water, a bilayer will always form when you put enough of the right type of phospholipids in water. This is very important to the overall function of the membrane because it keeps the structure intact. But, the head has no real say in who gets in and who stays out of the cell. |
If it’s helpful to see a visual of how the polar head is important in forming a bilayer, this video may be useful to you:
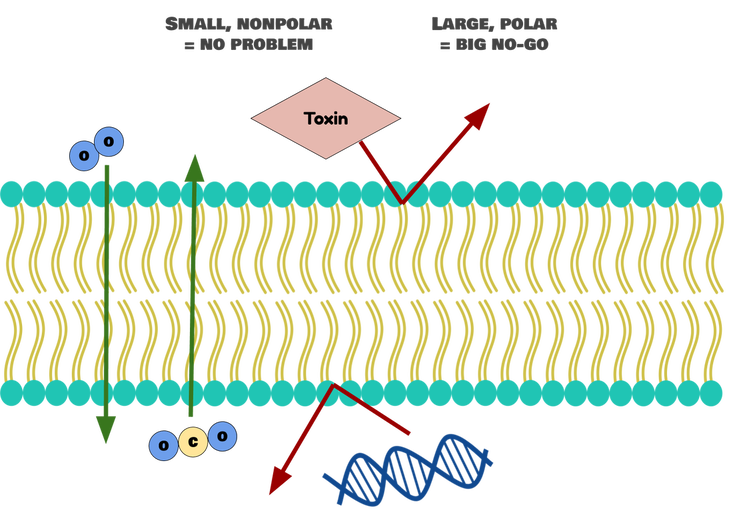
The tails are where the real function is at. Fatty acids are nonpolar, or hydrophobic: they like hanging out with each other, but not water (“phobic” means afraid of--so hydrophobic things are afraid of water). They will let small, nonpolar molecules through, because these molecules will be able to dissolve inside the membrane and sneak to the other side. Once these small, nonpolar molecules are in the bilayer, they just keep sliding through to the inside of the cell. Similarly, the hydrophobic interior of the bilayer blocks anything polar from getting through. Polar molecules are repelled by the lipids, so they just stay out.
Proteins
Now, you may be looking at that picture and thinking, “But, wait! Aren’t there some really good big and polar things that we want in our cells? Isn’t the membrane going to exclude those?” And, you’d be exactly right. The phospholipid bilayer is really exclusive, which means that it keeps out a lot of good things in addition to the bad things:
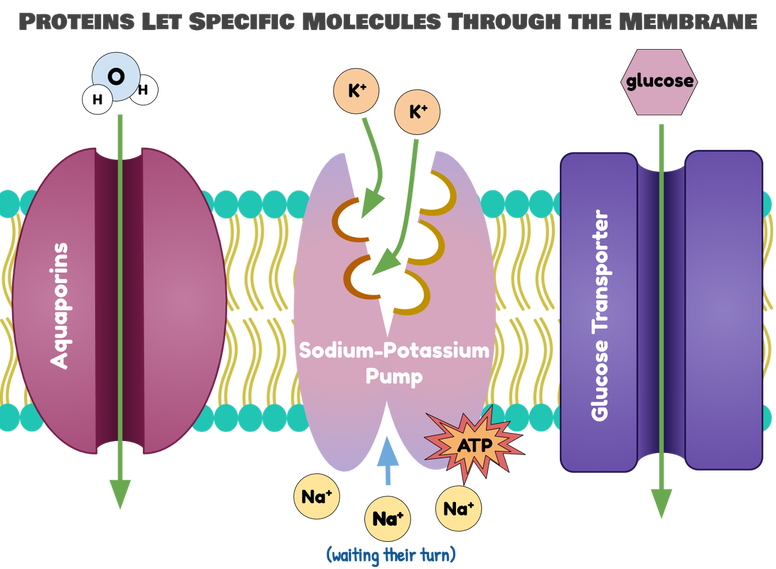
That’s where proteins come in! Integral proteins are proteins that go through the membrane to let specific molecules cross. Because proteins have highly specific structures and highly specific functions, they typically only let one (maybe two) things through. It’s the equivalent of getting put “on the list” at one of those swanky clubs where the bouncer didn’t want to let you in, or getting a special keycard to get let in the back gate.
Some examples of integral proteins include aquaporins (“water pores”), which let through water; glucose transporters, which let through glucose; and sodium-potassium pumps, which exchange sodium for potassium.
Integral proteins sometimes require an input of energy from a special cell energy molecule called ATP in order to work. You’ll learn a lot more about ATP in the next lesson, when we talk about cell respiration. For now, you can think of ATP as the battery that powers chemical reactions in the cell. Integral proteins also sometimes require a signal from another type of molecule (not the molecule it lets through) in order to work. For example, many glucose transporters only let glucose through if insulin is present. Insulin is a hormone that lets glucose into cells, lowering your blood sugar. You’ll learn a lot more about insulin when we talk about hormones in our next unit. For now, focus on the main idea that integral proteins can function in many ways, but they ultimately act as super-specific gates to let larger or more polar molecules (any molecule we want that can’t get through the bilayer) into the cell.
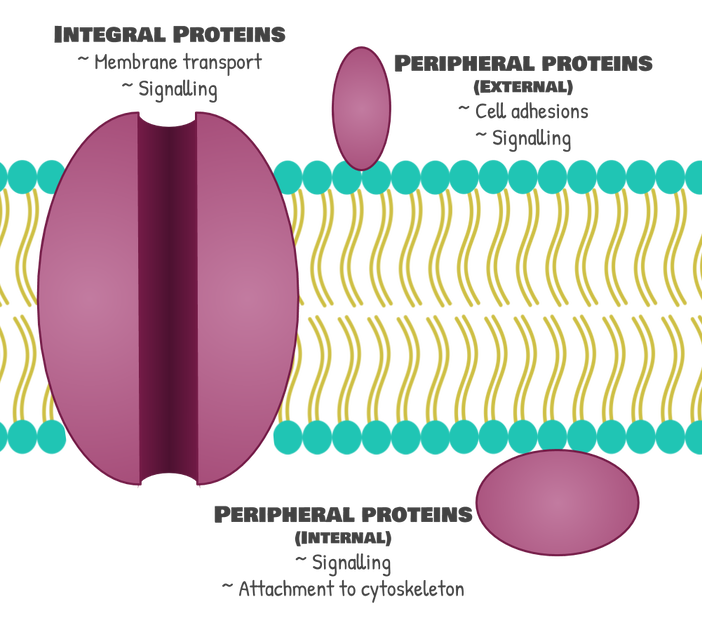
Integral proteins aren’t the only kind of proteins in the cell membrane. There are also peripheral proteins. Peripheral proteins can be on either the outside or the inside of the cell membrane, but they don’t go through the cell membrane. The peripheral proteins on the outside of the membrane are important for helping cells to “hold on” to one another through interactions among these proteins and are also really important for cell signalling, or letting cells talk to each other. Thinking about these functions, it makes sense why these proteins would need to be on the outside of the cell. The peripheral proteins on the inside of the cell are either used for signal transduction, which is passing on a signal from the outside of the cell to the inside of the cell, or for holding onto the cytoskeleton, which are proteins that give the cell structure.
Integral proteins sometimes require an input of energy from a special cell energy molecule called ATP in order to work. You’ll learn a lot more about ATP in the next lesson, when we talk about cell respiration. For now, you can think of ATP as the battery that powers chemical reactions in the cell. Integral proteins also sometimes require a signal from another type of molecule (not the molecule it lets through) in order to work. For example, many glucose transporters only let glucose through if insulin is present. Insulin is a hormone that lets glucose into cells, lowering your blood sugar. You’ll learn a lot more about insulin when we talk about hormones in our next unit. For now, focus on the main idea that integral proteins can function in many ways, but they ultimately act as super-specific gates to let larger or more polar molecules (any molecule we want that can’t get through the bilayer) into the cell.
Integral proteins aren’t the only kind of proteins in the cell membrane. There are also peripheral proteins. Peripheral proteins can be on either the outside or the inside of the cell membrane, but they don’t go through the cell membrane. The peripheral proteins on the outside of the membrane are important for helping cells to “hold on” to one another through interactions among these proteins and are also really important for cell signalling, or letting cells talk to each other. Thinking about these functions, it makes sense why these proteins would need to be on the outside of the cell. The peripheral proteins on the inside of the cell are either used for signal transduction, which is passing on a signal from the outside of the cell to the inside of the cell, or for holding onto the cytoskeleton, which are proteins that give the cell structure.
Carbohydrates
Carbohydrates do not serve any direct role in membrane transport, but they are important parts of the cell membrane. In the cell membrane, they are usually found attached to proteins. One of their main roles is to help with cell recognition.
For example, you may know that people have blood types, A, B, AB, or O, which help blood cells tell other cells what kind of cell they are. These blood types are actually just different types of carbohydrates on the cell surface. You may also know that germs have antigens on their surface, which is how our immune system knows that they’re germs. Many of these antigens are carbohydrates.
For example, you may know that people have blood types, A, B, AB, or O, which help blood cells tell other cells what kind of cell they are. These blood types are actually just different types of carbohydrates on the cell surface. You may also know that germs have antigens on their surface, which is how our immune system knows that they’re germs. Many of these antigens are carbohydrates.
Summary
Hopefully this lesson has impressed on you both the importance of the cell membrane and its role in regulating what goes in and out of the cell as well as the specific ways in which its structure—especially the special structure of phospholipids and the presence of integral proteins in the membrane—gives it its function.
This video gives a helpful recap of the structures addressed in this lesson:
This video gives a helpful recap of the structures addressed in this lesson:
This video also does a good job of explaining the same idea. You may might find it helpful to hear it explained just a little differently:
You should understand:
Basically, the cell membrane is super awesome, and you super need it in order to live. Thanks, cell membrane!
- The cell membrane is composed of phospholipids, whose unique structure (they are amphipathic) allows them to act as a fence to keep out everything but small, nonpolar molecules.
- The cell membrane has many integral proteins, which let specific molecules that we want into the cell. This lets larger or more polar molecules, like glucose and water, get in.
- The cell membrane has many other proteins and carbohydrates that play important roles, especially in cell signalling, cell adhesions (which help with cell and tissue structure), and cell recognition.
Basically, the cell membrane is super awesome, and you super need it in order to live. Thanks, cell membrane!
Learning Activity
Contributors: Alan Li, Emma Moulton