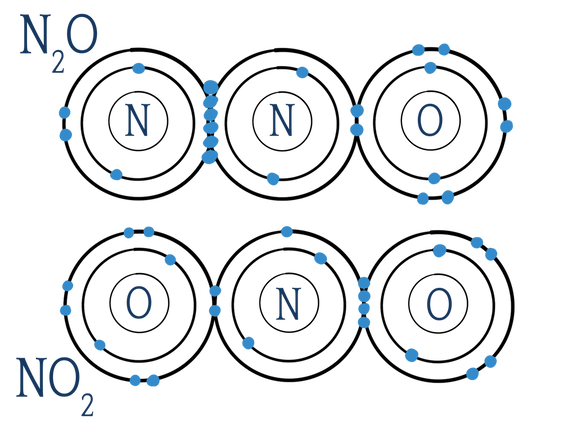Covalent Molecules
Without further adieu, I now present the main attraction: the mystical, the magical, the amazing and powerful, the one and only (drum roll please).... "Chemical Nomenclature of Covalent Molecules!" (Hold for roars and applause).
Long-form Naming
Long-form naming of covalent molecules isn't all that different than it is for naming ionic compounds. Like with ionic compounds, you first name the first element, and then the second, replacing the ending of the second with "-ide". However, with ionic compounds, two atoms can really only join in one way, because the charges have to balance. You would never find NaCl2 in nature. However, the atoms in covalent molecules are not charged. They can find different ways of sharing to make them most stable. For example, I could have N2O (also known as laughing gas), or I could have NO2 (an air pollutant, which is actually not particularly stable because nitrogen doesn’t have a full octet and is instead something called a free radical):
Just pop these suckers onto the beginning of your basic compound name (i.e., nitrogen oxide to nitrogen dioxide, for NO2, or dinitrogen monoxide, for N2O), and you have yourself a covalent compound. Beautiful, isn't it?
As with so many things that you've learned so far in this course, the process of naming covalent molecules is easier to learn by example than simply by reading about it. So, look through this chart and see if you can guess the names of the compounds, and check yourself by clicking to reveal the answer:
Knowledge is power! This table will tell you more about all the compounds you just saw.
BF₃ |
Smelly, colorless, toxic gas used as a building block for other boron compounds |
SF₆ |
- Makes your voice deep - Green house gas |
H₂O |
- Necessary for survival for almost all living things - Tasty beverage |
PCl₅ |
Adds chlorine to other things (useful in the lab) |
N₂H₄ |
- Colorless, flammable liquid - Antidepressant |
Molecular Formula
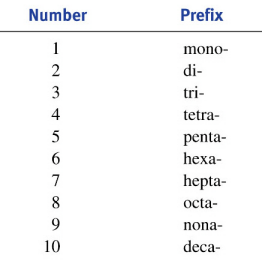
Pretty simple: just do the same thing, backwards. The advantage of using the Greek prefixes is that you don't have to figure out how many of each atom there should be: it just tells you. So, sulfur hexafluoride becomes SF6, and dihydrogen monoxide becomes H2O. It's that easy.
Common Names
In general, we chemists like to use the proper chemical name for things most of the time. That being said, you'd be hard pressed to find a chemist who refers to H2O as dihydrogen monoxide in their standard day-to-day conversations. They'll just say water. The same principle applies to a few other compounds as well, which are listed below. You should know these for your general knowledge.
Molecular Formula |
Common Name |
Chemical Name |
What it does |
H₂O |
water |
dihydrogen monoxide |
survival of living things |
NH₃ |
ammonia |
nitrogen trihydride |
smelly bathroom cleaner |
CH₄ |
methane |
carbon tetrahydride |
natural gas (and farts, ew!) |
O₃ |
ozone |
trioxygen |
atmosphere, protects earth from harmful UV rays |
Organic Molecules
Diatomic Molecules
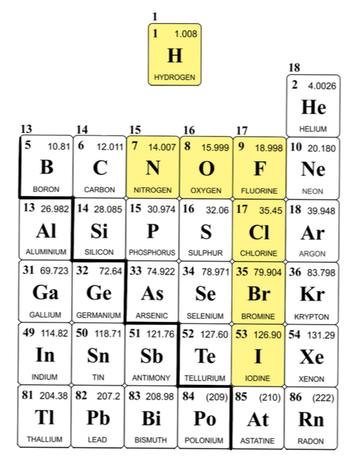
Interestingly enough, there are some molecules that are only found in nature as molecules, never as individual elements. The diatomic gases are among them. These molecules—H2, N2, O2, F2, Cl2, Br2, and I2—are only found in nature in pairs (i.e., bound to themselves). Because these molecules are quite common and are important in a number of chemical reactions, you should know them.
There are a few useful tricks for memorizing which elements are diatomic. One of my favorites is the mnemonic "HOFBrINCl" (pronounced "hof-brink-el"), which, as you'll notice, includes the atomic symbols of all of the diatomic elements. Another way to remember makes use of the periodic table, highlighting the "royal seven" elements (there are 7 diatomic elements in total, which look like a 7 on the periodic table. Hydrogen makes a crown.)
Summary
- How to write the long-form name of a covalent molecule, given the molecular formula. This requires you to know the standard greek prefixes for numbers.
- How to write the molecular formula of a covalent molecule, given the long-form name.
- The chemical formulas for the common elements water, ammonia, methane, and ozone.
- Which elements are only found in nature as diatomic elements.