EIS 4: 3D Molecular Models
In this activity, you will be making 3-dimensional (3D) molecular models out of candy and toothpicks. It is useful to be able to see models in 3D, because it helps you visualize how they will interact and react with other molecules, etc. It will give you hands-on practice with modeling covalent molecules. It’s also fun! We will also introduce a new idea called VSEPR (pronounced “ves-per”).
If you do not understand how to draw molecular models, go back and review that now. But, drawings are flat, and molecules are not. They are 3D just like you and me. And, just like you and me, the electrons like their space. So, they will spread out as much as possible, trying to get away from each other. This is called electrostatic repulsion or electron pair repulsion (the “EPR” of VSEPR; the rest is “valence shell”) and determines the shapes of molecules.
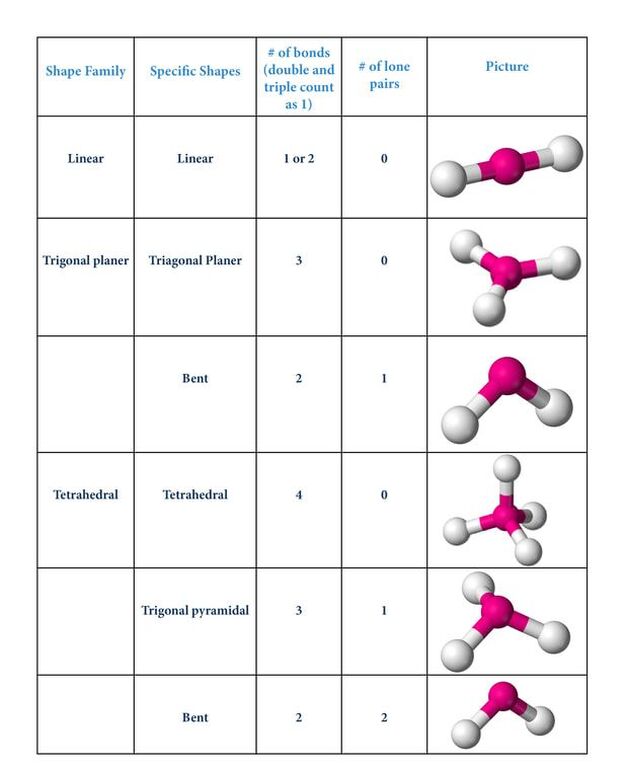
The table below gives the common shapes for simple molecules. They all are the way they are because the electrons are pushing each other apart.
If you do not understand how to draw molecular models, go back and review that now. But, drawings are flat, and molecules are not. They are 3D just like you and me. And, just like you and me, the electrons like their space. So, they will spread out as much as possible, trying to get away from each other. This is called electrostatic repulsion or electron pair repulsion (the “EPR” of VSEPR; the rest is “valence shell”) and determines the shapes of molecules.
The table below gives the common shapes for simple molecules. They all are the way they are because the electrons are pushing each other apart.
Materials
For this activity, you will need “sticks” (bonds) and “balls” (atoms) to make your models. You can use anything that will work for these, but here are some recommendations:
- Sticks: toothpicks, straws, uncooked spaghetti, twigs, etc.
- Balls: mini marshmallows, gummy bears, fruit snacks, Dots candy, or any squishy candy (it needs to be soft enough to easily put in and take out your “sticks”), styrofoam balls
Procedure
1. Print off (or hand copy) this chart and draw the Lewis diagrams for each structure. Don’t worry about 3D shape until step 2.
| EIS 4 Download! |
2. Consult the VSEPR chart above to determine what the shape of each molecule in your printed chart should be, based on the number of bonds and the number of lone electrons.
3. Build your structure out of whatever you’ve decided to use for sticks (bonds) and balls (atoms).
If you’re proud of your work, we want to see it! If you have an Instagram and the permission of your relevant responsible adult, share a photo or video with us @eons_learning, #MoleculesEons. When you’re done, answer the associated questions.
3. Build your structure out of whatever you’ve decided to use for sticks (bonds) and balls (atoms).
If you’re proud of your work, we want to see it! If you have an Instagram and the permission of your relevant responsible adult, share a photo or video with us @eons_learning, #MoleculesEons. When you’re done, answer the associated questions.
Content contributors: Emma Moulton