Phase Changes
As we started discussing in the last part of this lesson, even though matter exists in distinct phases called solids, liquids, or gases, they can also change between these phases due to changes in temperature and pressure. It’s like how you might change into a nicer person when its warm out and get really mean and grumpy when you’re too cold. Except, it’s not really at all like that. This is much more to do with the inherent factors of what defines a solid, liquid, and gas.
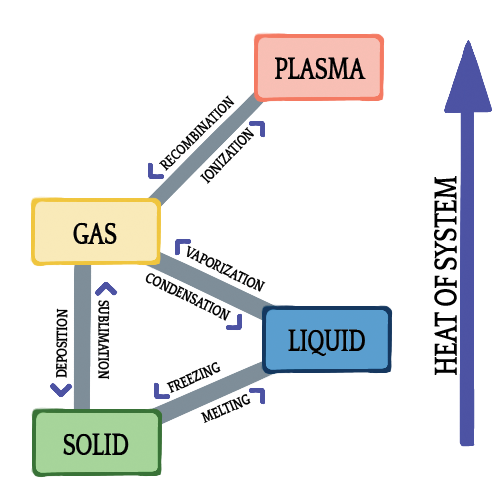
Phase change is a fancy term for changing states—i.e., going from a solid to a liquid or a liquid to a gas. Sometimes, a solid can skip over the liquid stage and go straight to a gas. The following diagram outlines the different types of phase changes:
Phase change is a fancy term for changing states—i.e., going from a solid to a liquid or a liquid to a gas. Sometimes, a solid can skip over the liquid stage and go straight to a gas. The following diagram outlines the different types of phase changes:
You hopefully already know the terms freezing and melting (aka fusion). You probably even recognize evaporation (vaporization) and condensation. But, did you know that you don't always have to go through the liquid stage to get from a solid to a gas, and vice versa? Yep, under certain conditions (such as a rapid change in temperature), sublimation and deposition can occur, skipping this middle liquid stage, as demonstrated in this video:
Sublimation is a change from solid to gas. The most common object that demonstrates this property is dry ice, or solid carbon dioxide. Deposition is the opposite of sublimation—it is when a gas turns into a solid. This is how snow forms in clouds. Even though they look like giant marshmallows, clouds are water vapor. When the temperature in the air suddenly becomes cool (≤ 32ºF), water vapor will condense and become a solid. It skips over the liquid phase because the sudden change in temperature (rapid removal of heat) does not allow time for the water vapor to condense into water (in this case, rain).
Phase Changes and Heat
How does phase change actually happen? Well, it has to do with energy. All matter has inherent energy about it: It’s not just sitting around, it’s doing stuff. We can see that in the equation E = mc². Matter is related to energy. If you transfer this energy—i.e., move it from a system to its surroundings or vice versa—in large enough amounts, a phase change can occur. This video does a good job of explaining how kinetic energy relates to the phases of matter:
In the case of a phase change, energy is in the form of heat. Heat, just like everything else in the universe, has a tendency to move from areas where there's a lot of it (i.e., where it's hot) to areas where there's not a lot of it (i.e., where it's cold). You can think of this like heat getting crowded at a party and just needing to escape. Also like everything else in the universe, heat will continue to move from hot to cold until it reaches equilibrium. This comes back to how molecules interact with each other: very fast moving molecules will speed up slow ones, and very slow molecules will slow down fast ones. This video does a good job of explaining the transfer of heat:
So, I'm sure you’re asking by this point, "Okay, cool, heat doesn't like to pile up in one spot. It likes to move around. Molecules balance each other’s energy. But what has any of this have to do with phase changes?"
Well, when you put an ice cube on a hot stove, it's going to melt (duh). This is because the stove (the surroundings) transfer heat to the ice cube (the system). This heat gives the system enough energy to break the bonds holding it in solid form, and it "collapses" into a liquid. The looser bonds give the molecules more freedom to move around, so we classify it as a liquid. Theoretically, if you keep adding heat to the system, these bonds will break apart, too, giving you a gas. Nifty, huh?
When something freezes, the same principle applies, only in reverse. Let's say you load an ice cube tray full of water into the freezer. In this situation, the system (the water) is warmer than the surroundings (the chilly freezer). Based on the laws of the universe, this means that heat will move out of the water and into the freezer. This has a negligible effect on the freezer (because it's so big), but takes enough energy from the water that the particles are too "lazy" to move much. This makes it rigid. Meanwhile, bonds reform, and you're left with a solid.
Well, when you put an ice cube on a hot stove, it's going to melt (duh). This is because the stove (the surroundings) transfer heat to the ice cube (the system). This heat gives the system enough energy to break the bonds holding it in solid form, and it "collapses" into a liquid. The looser bonds give the molecules more freedom to move around, so we classify it as a liquid. Theoretically, if you keep adding heat to the system, these bonds will break apart, too, giving you a gas. Nifty, huh?
When something freezes, the same principle applies, only in reverse. Let's say you load an ice cube tray full of water into the freezer. In this situation, the system (the water) is warmer than the surroundings (the chilly freezer). Based on the laws of the universe, this means that heat will move out of the water and into the freezer. This has a negligible effect on the freezer (because it's so big), but takes enough energy from the water that the particles are too "lazy" to move much. This makes it rigid. Meanwhile, bonds reform, and you're left with a solid.
The Heating Curve of Water
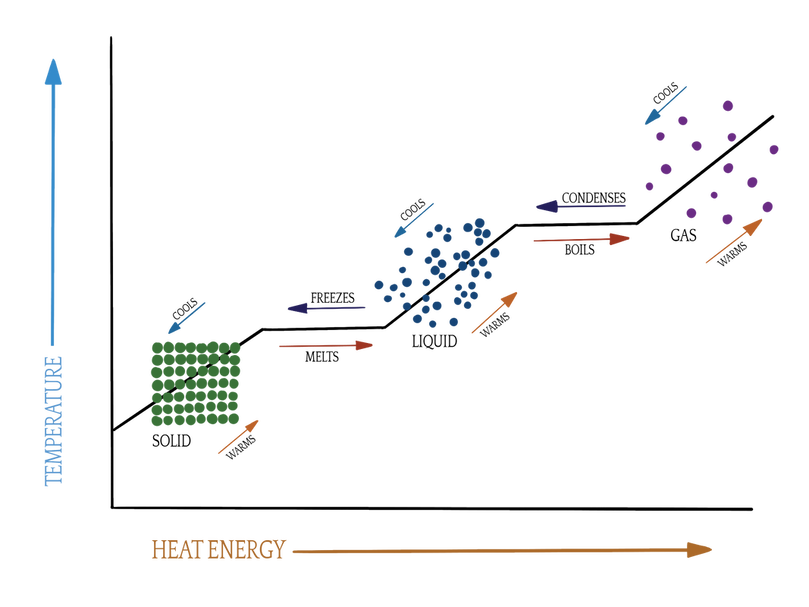
The heating curve of water is simply a nifty little graph that puts all of the information I just explained into one pretty picture. In theory, all substances would behave something like this, but for various reasons that doesn't always happen (or, if it does, it just happens at such extreme temperatures that the effect is negligible--for instance, helium condenses into a liquid at -270ºC, which is just 3º above the temperature at which all motion stops). Water's a pretty good model for phase change because all phases can be seen at relatively reasonable temperatures.
Here's what the heating curve of water looks like:
Here's what the heating curve of water looks like:
What does this mean, you ask? Well, let's look at it piece by piece.
First, lets look at the axes. The x (horizontal) axis is the heat of the system, or how much energy it has. So, as you move farther right, you are adding energy.
The y (vertical) axis is the temperature of the system. This is both what you can measure on a thermometer and a more conceptual idea that says that particles will move around more at higher temperatures (spoiler alert! You'll have to wait for lesson 7 for the rest.). So, as you move up, you're going up the scale on the thermometer, and particles are more free to move about as they choose.
At a low temperature and low heat (energy), water is a solid. It is ice. Particles don't move much, and it doesn't have much energy. But, as you start adding energy (say, with a space heater), the solid starts to warm up. The particles move around more, too. Then, something weird happens. You're still adding energy, but the temperature won't budge!
What's up with that?! Well this plateau happens at exactly 0ºC (32ºF), which you may recognize as the freezing point of water. At this temperature, water and ice are said to be at equilibrium*. On a molecular level, this plateau tells you that all of the energy is going into breaking apart those bonds holding ice together, so there's none leftover to move the temperature. This is called melting or fusion (or freezing, if you're going the other way).
Keep moving along the heat energy axis, and we're just warming up water. Whoa. That was fun. (Also, the molecules are moving around more).
Aaaand... we've reached another plateau. Yep, another equilibrium, only this time it's between liquid water and water vapor (gas). This occurs at 100ºC (212ºF), the boiling point of water. Just like at the first plateau, all of the energy you're adding in is going towards breaking apart bonds, and there's none left over to raise the temperature. (PS--this means that when you're boiling your spaghetti noodles, it doesn't really matter if the stove is on 9 or 1; as long as the water is still boiling, the temperature is exactly the same).
Made it! All of the water has been converted to gas. Whew! You deserve a trophy for all that energy you put in. Now you can heat up gas. What grand fun.
Keep heating, and maybe someday you'll get to plasma. But, fair warning, it needs to get pretty darn hot for that (think the Sun).
This video gives a great explanation of how the phase changes of water relate to what we see on the graph of its heating curve:
First, lets look at the axes. The x (horizontal) axis is the heat of the system, or how much energy it has. So, as you move farther right, you are adding energy.
The y (vertical) axis is the temperature of the system. This is both what you can measure on a thermometer and a more conceptual idea that says that particles will move around more at higher temperatures (spoiler alert! You'll have to wait for lesson 7 for the rest.). So, as you move up, you're going up the scale on the thermometer, and particles are more free to move about as they choose.
At a low temperature and low heat (energy), water is a solid. It is ice. Particles don't move much, and it doesn't have much energy. But, as you start adding energy (say, with a space heater), the solid starts to warm up. The particles move around more, too. Then, something weird happens. You're still adding energy, but the temperature won't budge!
What's up with that?! Well this plateau happens at exactly 0ºC (32ºF), which you may recognize as the freezing point of water. At this temperature, water and ice are said to be at equilibrium*. On a molecular level, this plateau tells you that all of the energy is going into breaking apart those bonds holding ice together, so there's none leftover to move the temperature. This is called melting or fusion (or freezing, if you're going the other way).
Keep moving along the heat energy axis, and we're just warming up water. Whoa. That was fun. (Also, the molecules are moving around more).
Aaaand... we've reached another plateau. Yep, another equilibrium, only this time it's between liquid water and water vapor (gas). This occurs at 100ºC (212ºF), the boiling point of water. Just like at the first plateau, all of the energy you're adding in is going towards breaking apart bonds, and there's none left over to raise the temperature. (PS--this means that when you're boiling your spaghetti noodles, it doesn't really matter if the stove is on 9 or 1; as long as the water is still boiling, the temperature is exactly the same).
Made it! All of the water has been converted to gas. Whew! You deserve a trophy for all that energy you put in. Now you can heat up gas. What grand fun.
Keep heating, and maybe someday you'll get to plasma. But, fair warning, it needs to get pretty darn hot for that (think the Sun).
This video gives a great explanation of how the phase changes of water relate to what we see on the graph of its heating curve:
Summary
This video gives a great overview of some of the most important topics covered in this lesson:
You should understand:
- The concept of heat and how it relates to kinetic energy of molecules.
- How adding heat relates to phase changes: as particles move more, they can “escape” their intermolecular interactions and go from solid to liquid to gas.
- How we graph heat changes: as we add heat, solids get warmer; then, all the energy goes into melting them into liquids, so the temperature doesn’t change; then liquids get warmer; then, all the energy goes into boiling the liquid, so the temperature doesn’t change; then, gases get warmer.
- The names of the phase changes between solids, liquids, and gases:
- Solid to liquid: melting, or fusion
- Liquid to gas: vaporization, or boiling
- Gas to liquid: condensation
- Liquid to solid: freezing
Learning Activity
Content contributors: Rebecca Deng, Emma Moulton