Change Over Time
As you’ve learned, our environment is a pretty wonderful place full of amazing, life-sustaining complexities, pretty lights, roaring winds, and, periodically, a few burning meteors. She’s a beast. And, like any wonderful and complicated lady, she’s changeable. In fact, she hasn’t always been this hospitable to life, and, if we’re not nice to her, she might not always be as nice to us as she has been. Here, we’ll learn about how the atmosphere has evolved from the early days of Earth and how it is continuing to change due to human activities.
Young Earth
We’ve already learned about one important reason that the Earth’s atmosphere is composed of the gases that it is: Gravity. Earth has the perfect amount of gravity to hold onto medium-to-heavy-weight gases like nitrogen, oxygen, and carbon dioxide, but not so much the tiny-to-small gases like methane, helium, and hydrogen that would make our atmosphere so dense that it would be suffocating. But, why does it contain the proportions of the gases that it does? We know it’s important that the atmosphere is only about 20% oxygen, or else it would be explosive:
… but why does the atmosphere contain only 20% oxygen? For the answer here, we have to take a look at the second reason that Earth’s atmosphere is the way it is: Earth’s lifeforms shape the atmosphere.
In the early days of Earth, when it was just a newborn little planet, long before the dinosaurs, Earth’s atmosphere (to our best guess) was composed of mainly a lot of water vapor, carbon dioxide, and methane, which was produced by puddles of magma and collisions of asteroids with the Earth’s surface. It was a pretty violent and chaotic place, which might have looked a lot like this:
In the early days of Earth, when it was just a newborn little planet, long before the dinosaurs, Earth’s atmosphere (to our best guess) was composed of mainly a lot of water vapor, carbon dioxide, and methane, which was produced by puddles of magma and collisions of asteroids with the Earth’s surface. It was a pretty violent and chaotic place, which might have looked a lot like this:
An artist’s rendition of early Earth
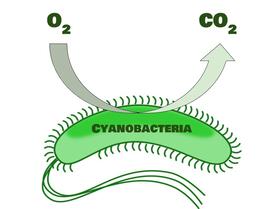
As life began to arise, mostly in the form of cyanobacteria, the atmosphere started to change. Cyanobacteria make energy through photosynthesis, which you may already know converts carbon dioxide to oxygen. (Plants do the same thing). This started to fill our atmosphere with oxygen and get rid of the excess carbon dioxide (and methane, by related processes) that made it too hot, steamy, and chaotic to be habitable by today’s more advanced lifeforms.
While these early cyanobacteria and other simple lifeforms were very important for starting off the early environmental changes that made more advanced life possible, the Earth didn’t really have enough oxygen for modern animal life until plants started to emerge and cover the entire earth. These plants, along with many bacteria, continued the atmospheric changes that filled our environment with enough oxygen to make animal (including human!) life possible.
This video provides a good recap of the atmosphere of early Earth and how it has changed. You’ll note it went through a few more cycles than we mentioned here, but the general trend is the proliferation of microbiotic and plant life that produced oxygen:
A Balanced Atmosphere
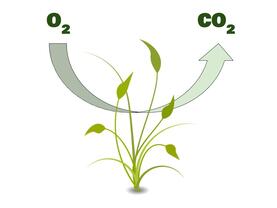
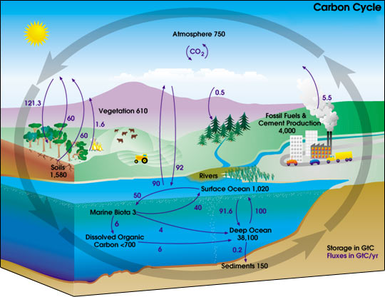
Since animal life has arisen on Earth, the atmosphere hasn’t really changed much. There are two important cycles at play here, which help to maintain the perfect balance of oxygen, nitrogen, and carbon dioxide: the carbon cycle and the nitrogen cycle. In the carbon cycle, animals use up oxygen and make carbon dioxide, while plants use up carbon dioxide and make oxygen. This keeps the levels of those two gases stable in the atmosphere:
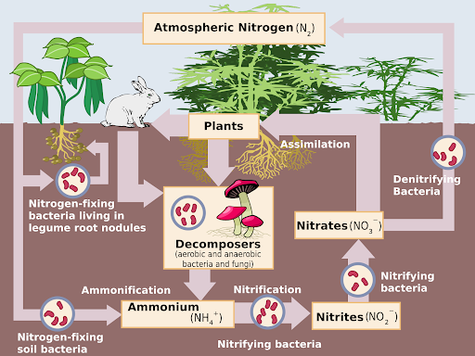
Meanwhile, many bacteria, especially soil-dwelling bacteria, regulate the amount of nitrogen in the atmosphere through the nitrogen cycle:
Don’t worry too much about the precise details of those cycles: we’re mainly interested in you understanding that the Earth’s atmosphere remains stable when all of Earth’s lifeforms work together symbiotically to maintain that atmosphere. As long as there’s a perfect balance of all of these different forms of life, the atmosphere won’t change at all. Life will continue to thrive on Earth.
Climate Change Due to Human Intervention
As great as these carbon and nitrogen cycles are if just left up to the forces of nature, unfortunately, human intervention has caused the amount of carbon dioxide produced to be significantly greater than its consumption. There are two main forces at work here:
Increased Carbon Dioxide Emissions. The burning of fossil fuels (oil, gasoline, and coal, among others) and, to a lesser extent, wood—which are the primary energy sources currently in use by humans—has significantly increased since the Industrial Revolution. This is approximately the past 100 years. As human society has become more technologically advanced, our energy demand has increased significantly, and, though we have started to make some steps toward relying on greener energy sources like solar, wind, and nuclear power, we have mostly met this demand by burning fossil fuels.
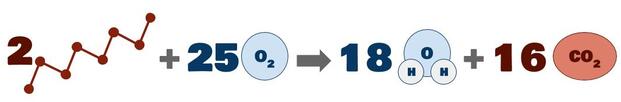
Combustion reactions produce significant amounts of carbon dioxide and energy. Consider the combustion of gasoline, which is a mixture of hydrocarbons averaging about 8 carbons in length:
Increased Carbon Dioxide Emissions. The burning of fossil fuels (oil, gasoline, and coal, among others) and, to a lesser extent, wood—which are the primary energy sources currently in use by humans—has significantly increased since the Industrial Revolution. This is approximately the past 100 years. As human society has become more technologically advanced, our energy demand has increased significantly, and, though we have started to make some steps toward relying on greener energy sources like solar, wind, and nuclear power, we have mostly met this demand by burning fossil fuels.
Combustion reactions produce significant amounts of carbon dioxide and energy. Consider the combustion of gasoline, which is a mixture of hydrocarbons averaging about 8 carbons in length:
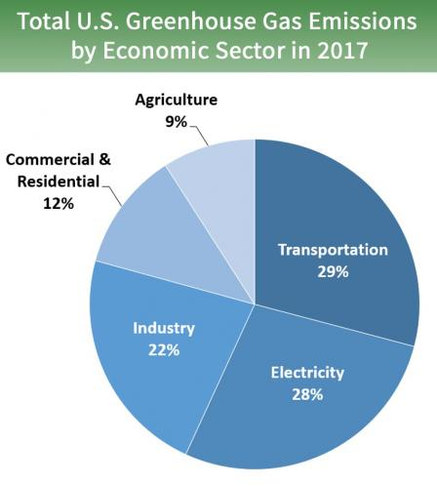
In layman's terms, almost 20 pounds of carbon dioxide emissions are produced per gallon of gasoline burned! Multiply that by all the cars on the road every day, and then consider that all transportation (including airline travel, train travel, cargo shipment, long-haul trucking, and so on) account for less than a third of total CO₂ emissions, and you can start to see why the amount of CO₂ in our atmosphere is trending upward so rapidly.
Deforestation. While an increase in carbon dioxide emissions seems to be by far the greatest contributor to the increase in carbon dioxide in our atmosphere, it doesn’t help that, all the while we’ve been pumping carbon dioxide into the atmosphere, we’ve been cutting down trees en masse. As you’ve learned, plants use carbon dioxide and make oxygen. Less carbon dioxide used + more carbon dioxide made = a whole lotta carbon dioxide in the atmosphere.
Next question: Why is this increase in carbon dioxide bad?
Well, an increase in carbon dioxide in our atmosphere causes a runaway greenhouse effect—like the one that makes Venus’ atmosphere so dense and toxic that even highly specialized space robots have a difficult time surviving it. The greenhouse effect is where the atmosphere traps in heat from the sun, like you might have noticed happens if you’ve ever been in a greenhouse or any other room with a lot of closed windows on a sunny day. A little bit of greenhouse effect makes our planet nice and warm for human habitation. A lot of greenhouse effect makes our planet inhospitably warm for many species, starting with those that live in cold climates, like penguins and polar bears, and, as the greenhouse effect worsens, making the planet too hot for even humans.
If the greenhouse effect continues to worsen, it will eventually cause an increase in water vaporization from our oceans. This results in more water vapor—another greenhouse gas—in our atmosphere. This causes more greenhouse effect, which causes more water to be built up in our atmosphere, and so on and so on in a vicious cycle that is, based on our current knowledge, impossible to disrupt (hopefully someone smart, like you, can come up with a way to completely reverse it before it’s a major problem). The point at which water significantly starts to vaporize because of the greenhouse effect is referred to as the point of no return.
This video explains the greenhouse effect:
Next question: Why is this increase in carbon dioxide bad?
Well, an increase in carbon dioxide in our atmosphere causes a runaway greenhouse effect—like the one that makes Venus’ atmosphere so dense and toxic that even highly specialized space robots have a difficult time surviving it. The greenhouse effect is where the atmosphere traps in heat from the sun, like you might have noticed happens if you’ve ever been in a greenhouse or any other room with a lot of closed windows on a sunny day. A little bit of greenhouse effect makes our planet nice and warm for human habitation. A lot of greenhouse effect makes our planet inhospitably warm for many species, starting with those that live in cold climates, like penguins and polar bears, and, as the greenhouse effect worsens, making the planet too hot for even humans.
If the greenhouse effect continues to worsen, it will eventually cause an increase in water vaporization from our oceans. This results in more water vapor—another greenhouse gas—in our atmosphere. This causes more greenhouse effect, which causes more water to be built up in our atmosphere, and so on and so on in a vicious cycle that is, based on our current knowledge, impossible to disrupt (hopefully someone smart, like you, can come up with a way to completely reverse it before it’s a major problem). The point at which water significantly starts to vaporize because of the greenhouse effect is referred to as the point of no return.
This video explains the greenhouse effect:
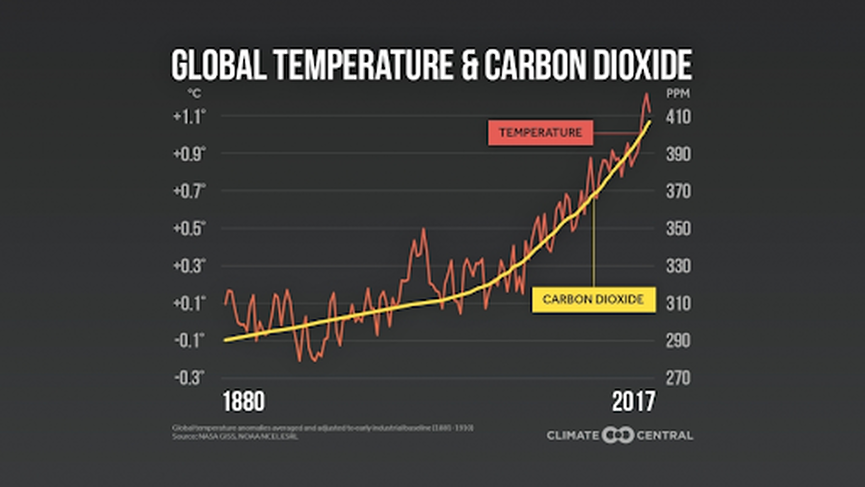
If you still question the connection between carbon dioxide and climate change, consider this graph:
Of course, correlation is not causation, but, given the proven mechanism which supports causation through a greenhouse effect, the evidence for carbon dioxide causing climate change is very strong.
Climate change, or global warming, is not just about our beaches being warmer, having fewer snow days, or polar bears dying. It has significant implications for the way humans live, which is approaching faster than we’d hope. This video discusses more of the implications of climate change:
Climate change, or global warming, is not just about our beaches being warmer, having fewer snow days, or polar bears dying. It has significant implications for the way humans live, which is approaching faster than we’d hope. This video discusses more of the implications of climate change:
Fortunately, there are still steps we can take to combat this major climate crisis. This includes some everyday changes to the way we live our lives, like reducing our usage of single-use plastics, switching to public transit or electric vehicles, and reducing our everyday energy usage. But, in order to really fix this problem, massive change needs to occur on an international level in the way that we think about and use energy. For example, we need better, more efficient ways of making cleaner energy (solar, wind, water, and nuclear). With all of our energy demands, it’s a lot to ask to completely get rid of fossil fuels when the alternatives tend to be more expensive and not as good. So, we need scientists and engineers—possibly like you!—working to create better green energy. There is also a lot of political change that needs to occur in terms of regulating and enforcing regulations around what types of energy big factories can and can’t use. Finally, we need a way to reverse the climate change that has already occurred by removing carbon dioxide from our atmosphere. While planting a lot of trees, for example, will help with this, we need bright thinkers—possibly like you!—coming up with better, more efficient ways of stopping and reversing the damage caused by carbon dioxide and climate change.
Summary
Hopefully, this lesson has given you some appreciation for how our atmosphere has evolved over time, the mechanisms that exist that keep it in perfect balance for sustaining all kinds of life, and the importance of keeping it habitable by reducing carbon dioxide emissions and cleaning up our atmosphere. As scary as climate change may be—and it is very scary!—with the combined everyday actions of all of us, plus scientific advances from the best and the brightest minds—that could mean you!—on the job, we can make our planet as wonderful and life-sustaining as we know it can be for eons to come. Climate change is a big problem, which makes it a problem worth fixing.
You should understand:
You should understand:
- That early lifeforms, including cyanobacteria and eventually plants, shaped the Earth’s atmosphere to increase the amount of oxygen in it and make Earth habitable for life today.
- That the diversity of lifeforms on Earth using different mechanisms for producing energy is what keeps the atmosphere the same, through the carbon and nitrogen cycles. (You do not need to know the details of these cycles).
- That human intervention, especially increased carbon dioxide emissions through burning fossil fuels, has, through the greenhouse effect, caused global warming/climate change, which is a major crisis that we must take major steps toward correcting.
Learning Activity
Content contributors: Emma Moulton