Macromolecules for Energy: Carbohydrates and Lipids
What are Macromolecules?
As we learned earlier in this lesson, macromolecule means “big molecule”. We especially use it to refer to the large polymers that perform most major functions in our bodies.
You’ve probably heard of a lot of these macromolecules before. They are: carbohydrates, lipids, proteins, and nucleic acids. They are made up primarily of carbon, hydrogen, and oxygen atoms, which makes them organic compounds.
Right now, we’re going to focus on carbohydrates and lipids. Both of these molecules provide energy for our bodies, in addition to a few other functions that we’ll talk about. We’ll talk about proteins and nucleic acids later in this lesson.
You’ve probably heard of a lot of these macromolecules before. They are: carbohydrates, lipids, proteins, and nucleic acids. They are made up primarily of carbon, hydrogen, and oxygen atoms, which makes them organic compounds.
Right now, we’re going to focus on carbohydrates and lipids. Both of these molecules provide energy for our bodies, in addition to a few other functions that we’ll talk about. We’ll talk about proteins and nucleic acids later in this lesson.
Why Do We Need Energy?
We’ll talk a lot more about how our bodies make and use energy when we talk about cell respiration. Cell respiration is how our bodies turn energy from food into usable energy for our bodies. Our bodies use this energy to power chemical reactions, which use proteins.
Check out this video for a basic overview of the importance of energy in our bodies:
Check out this video for a basic overview of the importance of energy in our bodies:
Carbohydrates for Quick Energy
You’ve probably heard of carbohydrates, or “carbs,” in reference to your food. Their main role is to provide us with short-term, quick-acting energy.
Carbohydrates, like in cereal, give you fast-acting energy.
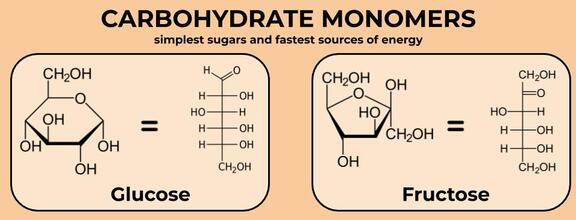
Technically, not every carbohydrate is a macromolecule, because some of them can be quite small. These small carbohydrates include the monomers glucose and fructose. When a monomer is a sugar, we call it a monosaccharide (“saccharide” is a fancy word for sugar). These molecules--especially glucose--are our body’s preferred energy source. They provide the easiest and fastest-acting source of fuel, because our body’s main mechanism for making energy, cell respiration, is really well designed for breaking down glucose and fructose. In fact, almost all living things prefer to use glucose as fuel. We’ll learn more about cell respiration later. (As a side note, even though our bodies are best at breaking down glucose and fructose, it can also get energy from fat and, to a lesser extent, protein--that’s just a slightly more complicated process).
|
Glucose and fructose are made up of rings of carbons and an oxygen. Both have 6 carbons, but fructose forms a 5-membered ring because of the placement of the double-bonded oxygen. Importantly, these molecules can be found in a linear (line) pattern, rather than a ring. They’re the same molecule, just in a slightly different arrangement. These aren’t the only two kinds of monosaccharides, but they’re the most common in biology.
|
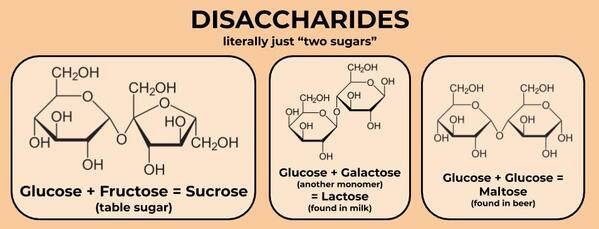
There are also slightly larger molecules called disaccharides (literally “two sugars”), which are two monomers linked together. The most common disaccharide (for humans) is sucrose, which is a glucose linked to a fructose. This is the kind of sugar that you can buy at the grocery store and that is usually used in cooking. We break it down into energy by breaking apart the bond between the glucose and fructose and then getting energy out of glucose and fructose through cell respiration.
Disaccharides are made up of two monomeric sugars, like glucose and fructose, linked together.
Sucrose, lactose, and maltose are some of the most common, at least as far as stuff humans eat.
Sucrose, lactose, and maltose are some of the most common, at least as far as stuff humans eat.
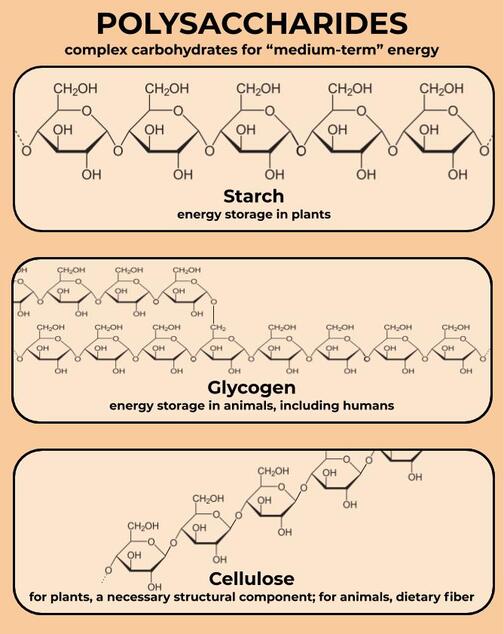
Much larger carbohydrates--the true macromolecules--include starch, glycogen, and cellulose. They are all big chains of glucose linked together in slightly different ways. Starch and glycogen are both long chains of glucose used to store energy for later--not quite “long-term” energy, which is the role of fat, but definitely “medium-term” energy.
Glycogen is found in animals, including humans. When you eat a lot of sugar, your liver turns glucose into glycogen to store it for later and lower your blood sugar. When you’re running low on sugar--especially during exercise--your liver turns glycogen back into glucose to make sure your cells still have enough energy.
Starch has basically the same job as glycogen, but it is found in plants. It is found in a lot of foods we eat, like wheat, rice, potatoes, carrots, beets, and other grains and root vegetables. Because these foods take a little bit longer to break down than simple sugars, they won’t give you the same “crash” that you might get after eating a candy bar or drinking a soda. This is especially true if those foods are rich in cellulose, or dietary fiber.
Our bodies can’t break down cellulose, so we don’t use it for energy, but it is very important for plants because it helps them hold their structure. We’ll learn a bit more about cellulose when we talk about plant cells. We also need it in our diets, in order to keep our digestive systems moving. Eat plants!
Glycogen is found in animals, including humans. When you eat a lot of sugar, your liver turns glucose into glycogen to store it for later and lower your blood sugar. When you’re running low on sugar--especially during exercise--your liver turns glycogen back into glucose to make sure your cells still have enough energy.
Starch has basically the same job as glycogen, but it is found in plants. It is found in a lot of foods we eat, like wheat, rice, potatoes, carrots, beets, and other grains and root vegetables. Because these foods take a little bit longer to break down than simple sugars, they won’t give you the same “crash” that you might get after eating a candy bar or drinking a soda. This is especially true if those foods are rich in cellulose, or dietary fiber.
Our bodies can’t break down cellulose, so we don’t use it for energy, but it is very important for plants because it helps them hold their structure. We’ll learn a bit more about cellulose when we talk about plant cells. We also need it in our diets, in order to keep our digestive systems moving. Eat plants!
This video gives a good overview of the role of carbohydrates in providing energy to our bodies:
If you’re interested in learning more about how our bodies use the carbs we eat, check out this video:
Lipids for Sustained Energy
The term lipids broadly refers to long chains of carbon that don’t dissolve in water. There are three main kinds of lipids in our body, each of which has their own function.
The type of lipid we use for energy is fat, also known as triglycerides. Fat is used for long-term energy, especially energy storage. Our bodies can use fat for energy, but not as easily as sugar, so it will usually use up the available sugar before it starts metabolizing fat. Metabolizing means “doing cell respiration on.”
When we eat a lot of sugar, our bodies will start metabolizing it and then turn that into fat before we actually get much energy out of the sugar. In simpler terms: if you eat a lot of sugar, you’ll get fat. Why? Because if there’s plenty of sugar around, our bodies have evolved to store it for the future, in case we run out of food and need that energy later. The fat under your skin also serves the important function of keeping us warm. Remember: Fat is for long-term energy storage.
The type of lipid we use for energy is fat, also known as triglycerides. Fat is used for long-term energy, especially energy storage. Our bodies can use fat for energy, but not as easily as sugar, so it will usually use up the available sugar before it starts metabolizing fat. Metabolizing means “doing cell respiration on.”
When we eat a lot of sugar, our bodies will start metabolizing it and then turn that into fat before we actually get much energy out of the sugar. In simpler terms: if you eat a lot of sugar, you’ll get fat. Why? Because if there’s plenty of sugar around, our bodies have evolved to store it for the future, in case we run out of food and need that energy later. The fat under your skin also serves the important function of keeping us warm. Remember: Fat is for long-term energy storage.
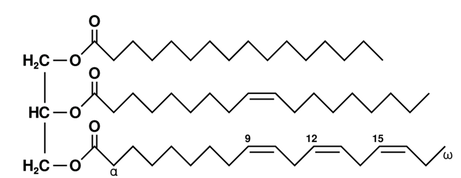
Fat is made up of three fatty acids--long chains of carbons with that
“--COOH” group on theend--hooked up to a glycerol backbone.
“--COOH” group on theend--hooked up to a glycerol backbone.
To see why we get so much energy out of fat versus sugar, just look at how many carbon-carbon bonds there are. To simplify our metabolism a lot, each of these carbon-carbon bonds releases a lot of usable energy when broken by our bodies. By comparison, glucose only has 6 carbon-carbon bonds, so we don’t get nearly as much energy out of it.
If you’re interested in seeing the process of breaking down fat and how this manages to get so much more useful energy out of it versus breaking down sugar, you may find this video interesting. Note that you do not have to understand any of this process for this class, and it might be a little advanced for your stage of learning. But some people might find it helpful just to have a visual:
If you’re interested in seeing the process of breaking down fat and how this manages to get so much more useful energy out of it versus breaking down sugar, you may find this video interesting. Note that you do not have to understand any of this process for this class, and it might be a little advanced for your stage of learning. But some people might find it helpful just to have a visual:
If you’re interested in learning more about how our bodies use the fats we eat, check out this video:
Other Jobs of Carbohydrates and Lipids
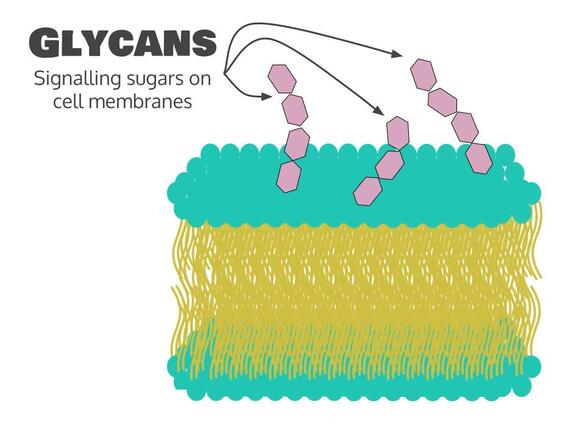
While the main role of carbohydrates and lipids in our body is to provide energy, they are also good at lots of other things. For example, certain carbohydrates called glycans are important for communication between cells. We call this signalling. Signalling is mostly the job of proteins, so we’ll talk more about signalling pathways when we talk about proteins.
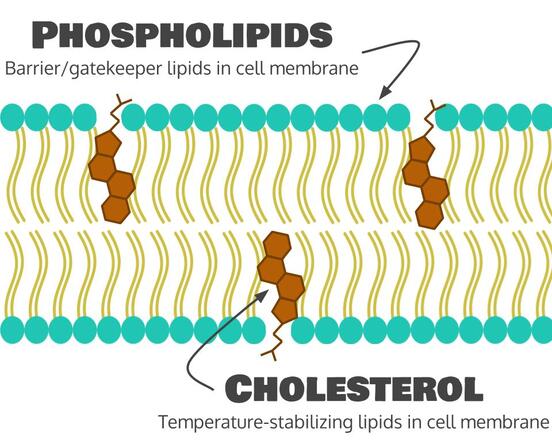
There are also two more important jobs of lipids in the body. The first is to make up cell membranes. We’ll learn more about cell membranes later in this unit. They’re basically the walls and the gates of our cells, deciding what goes in and what goes out. There are two main types of lipids that make up our cell membranes. The main ones are called phospholipids, which make up the majority of our cell membrane and are the main barrier/gatekeeper. The second is cholesterol, a sterol (ring lipid), which is found in cell membranes and helps to keep our cells intact in hot or cold temperatures.
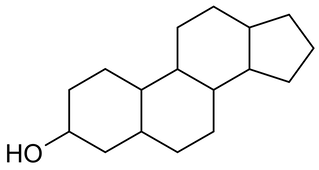
Sterols are lipids with a 4-ring structure, which are very similar to steroids. Both steroids and sterols are often signalling molecules (molecules used for communication between cells). In humans, two main steroids are estrogen, which is responsible for the development of female sexual characteristics, and testosterone, which is responsible for the development of male sexual characteristics.
Steroids and sterols have 4 rings
Both carbohydrates and lipids have a major function of providing energy for the body. You probably already know that we need energy, but it’s alright if you can’t totally explain what these means in terms of chemistry just yet. Next sublesson, we’ll talk more about what these molecules are actually providing energy for, and, when we learn about cell respiration, we’ll talk more about how our bodies actually translate intangible “energy” into an actual chemical reaction.
In other words, we don’t expect you to really fully understand the exact roles of carbohydrates and lipids just yet, because we haven’t seen them in action yet! For now, we’re just introducing you to the terms and the general functions so that when we see “carbohydrates” and “lipids” again, you’ll be ready to understand what they are and what they’re doing. It’s like meeting a new person for the first time: You go through all the niceties of “What’s your name? Where are you from? What do you do?,” but you probably won’t remember much of that unless you see that person again and again and continue to get to know them better.
So, we’ll see our friends again. For now, you should understand:
In other words, we don’t expect you to really fully understand the exact roles of carbohydrates and lipids just yet, because we haven’t seen them in action yet! For now, we’re just introducing you to the terms and the general functions so that when we see “carbohydrates” and “lipids” again, you’ll be ready to understand what they are and what they’re doing. It’s like meeting a new person for the first time: You go through all the niceties of “What’s your name? Where are you from? What do you do?,” but you probably won’t remember much of that unless you see that person again and again and continue to get to know them better.
So, we’ll see our friends again. For now, you should understand:
- Cells need energy to power the chemical reactions of life. Energy comes in 3 levels of storage:
- Simple sugars or monosaccharides, which are carbohydrates, provide immediate energy that can’t be stored for long.
- Polysaccharides, like glycogen and starch, which are also carbohydrates, provide temporary storage and “medium-term” energy.
- Triglycerides or fats, which are lipids, provide long-term storage and sustainable energy.
- Simple sugars or monosaccharides, which are carbohydrates, provide immediate energy that can’t be stored for long.
- Both carbohydrates and lipids have other functions unrelated to energy. For example:
- Glycans are carbohydrates involved in signalling, or communication between cells.
- Phospholipids and cholesterol are lipids that are found in the cell membrane.
- Steroids are lipids that are involved in signalling.
- Glycans are carbohydrates involved in signalling, or communication between cells.
- How to identify glucose, fructose, disaccharides, polysaccharides, triglycerides, phospholipids, and steroids/sterols based on their chemical structures.
Summary
You should understand:
- That ionic bonds involve a positively-charged metal cation donating electrons to a negatively-charged nonmetal anion.
- That positively-charged metal cations and negatively-charged nonmetal anions are held together by electrostatic forces, or “opposites attract.”
- How to find charge based on an ion’s location on the periodic table: Metals lose their valence electrons to become positively charged, and nonmetals gain valence electrons to achieve a full octet.
- That ionic bonds can hold together multiple ions, but the overall charge must add up to zero. You should also be able to figure out how many of each type of ion is required, based on their charge.
Learning Activity
Contributors: Allan Wu and Emma Moulton