Ionic Bonding
Ionic Bonding: Giving and Receiving
Atoms love to be stable. It makes them happy. It completes them. It means they get to stop spending their lives in a high energy state of having too much or not enough and instead find contentment in a low-energy, mutually beneficial partnership. In order to be stable, they need a full octet. Since not everyone is so lucky to just naturally have that, atoms need to interact with each other and form chemical bonds. Like in this beautiful story of two atoms finding each other and forming a harmonious ionic bond:
In more scientific terms, atoms need to either donate and accept or share valence electrons to attain a stable configuration. Donating and accepting electrons forms an ionic bond; sharing electrons forms a covalent bond.
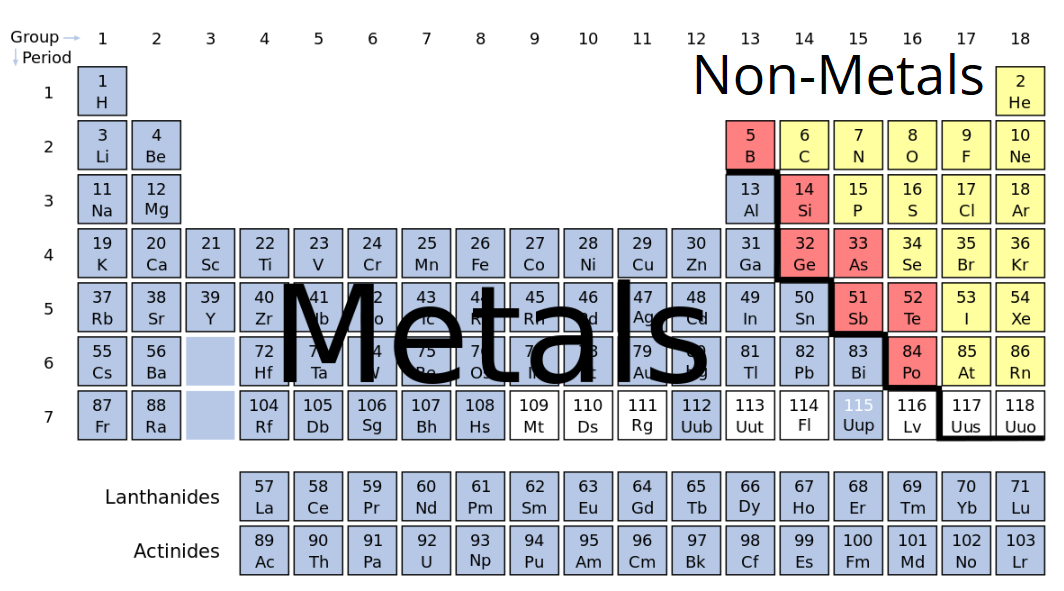
Ionic bonding occurs when a positively charged metal ion (called a cation), which is a metal atom that gave up an electron, and a negatively charged nonmetal ion (called an anion), which is a nonmetal atom that picked up an electron, get together. (“Lets get together, yeah, yeah, yeah!”) The most famous example of this is sodium chloride (aka NaCl, aka table salt):
Recall that metals are malleable, shiny, and ductile, and fall to the left of the zig-zaggy separation line on the Periodic Table. Nonmetals are either gases or brittle, dull solids; they fall to the right of the zig-zaggy separation line on the Periodic Table.
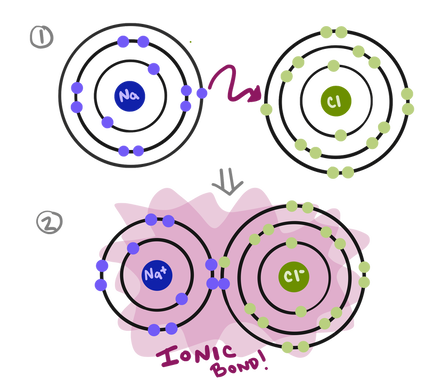
In sodium chloride, a sodium atom loses an electron to become a positively charged sodium ion. A chlorine atom gains an electron to become a negatively charged chloride ion. If we looked at the Bohr models of this interaction, it would look something like this:
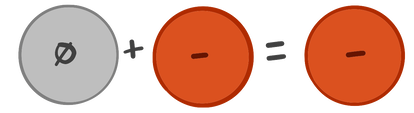
When chlorine picks up an electron, it becomes negatively charged. If it seems weird that adding an electron would give it a minus charge, you should remember that electrons are negatively charged. Adding a negative gives you a negative.
When chlorine picks up an electron, it becomes negatively charged. If it seems weird that adding an electron would give it a minus charge, you should remember that electrons are negatively charged. Adding a negative gives you a negative.
Since opposites attract (due to the electrostatic forces between them), these positive and negative ions then stick to each other like the two ends of a magnet. Everybody is happy and stable with the right number of electrons, and they’re happy together!!! How nice.
Once again, step-by-step:
- Regular old sodium is highly reactive (unstable). Since everything in the universe wants to be stable, it will ditch an electron to become more stable and have a full octet. Now it is a positively charged sodium ion.
- Chlorine gains an electron, making it more stable because it has a full octet. It becomes negatively charged.
- Because opposites attract, the positive sodium ion and the negative chloride ion stick together, kind of like two ends of a magnet. This is the electrostatic force at work.
Lattice Structures
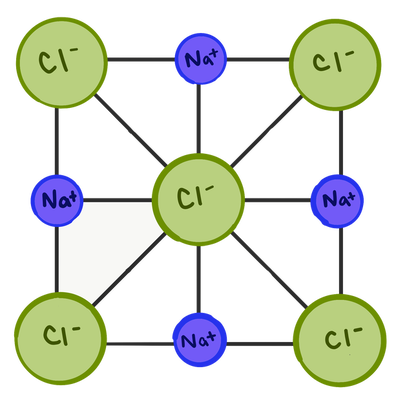
This is actually happening between many sodium and chloride ions all at the same time. Sodium #1 is attracted to chloride #2, which is attracted to sodium #3 and so on; this makes an elaborate pattern called a lattice structure. When enough ions get together, you can see a crystal form. It looks something like this:
Lattice structure of sodium chloride. Note that many sodiums and many chlorines are kind of all bonded to each other (but still in a 1:1 ratio).
Salt crystal
Ionic Bonding with Charges Higher than 1
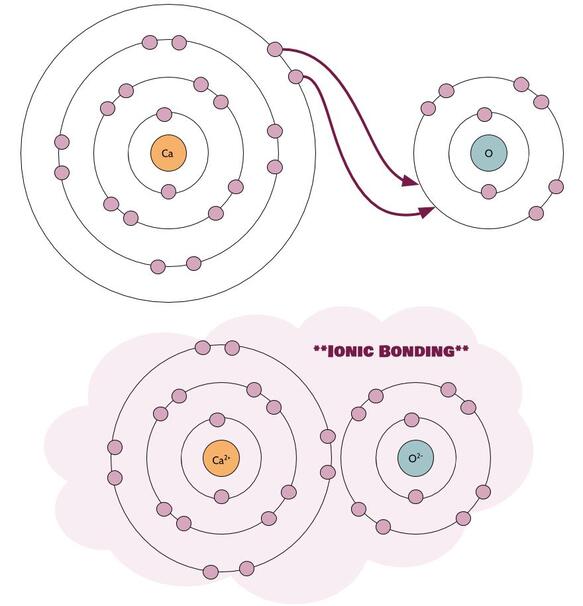
Sometimes, atoms need to lose more than one electron in order to become most stable. For example, calcium has two valence electrons, and it needs to give up both in order to get a full octet. There are then two options.
Option 1: It can end up attracting one thing with a 2⁻ charge, like in the case of calcium oxide (CaO):
Option 1: It can end up attracting one thing with a 2⁻ charge, like in the case of calcium oxide (CaO):
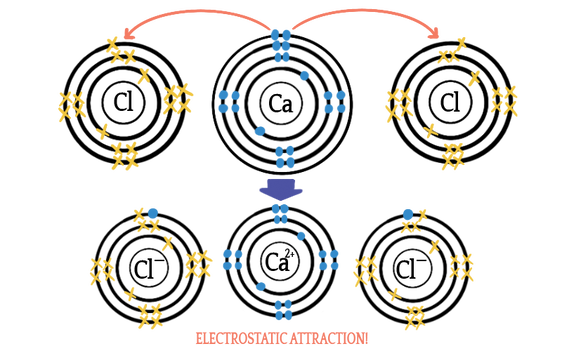
Option 2: It can attract 2 things that each have a 1⁻ charge, like in the case of calcium chloride (CaCl₂):
Now calcium has two best friends. Yay!
When you are dealing with ionic compounds, the general rule is that the charges have to add up to zero. In the case of calcium oxide (2⁺) + (2⁻) = 0. In the case of calcium chloride: (2⁺) + 2•(1⁻) = 0. So, you can figure out how many of each ion are in any ionic compound just by knowing their charges. We'll practice more with this when we talk about chemical nomenclature.
There are also these cool things called polyatomic ions. Basically it's just a covalent molecule (something you’ll learn about later!) that has a charge. This allows it to form ionic bonds with other charged ions. Pretty nifty!
And that is ionic bonding in a nutshell! Just having, and sharing, and giving, and receiving, and receiving, and giving, and bonding.
When you are dealing with ionic compounds, the general rule is that the charges have to add up to zero. In the case of calcium oxide (2⁺) + (2⁻) = 0. In the case of calcium chloride: (2⁺) + 2•(1⁻) = 0. So, you can figure out how many of each ion are in any ionic compound just by knowing their charges. We'll practice more with this when we talk about chemical nomenclature.
There are also these cool things called polyatomic ions. Basically it's just a covalent molecule (something you’ll learn about later!) that has a charge. This allows it to form ionic bonds with other charged ions. Pretty nifty!
And that is ionic bonding in a nutshell! Just having, and sharing, and giving, and receiving, and receiving, and giving, and bonding.
Summary
You should understand:
- That ionic bonds involve a positively-charged metal cation donating electrons to a negatively-charged nonmetal anion.
- That positively-charged metal cations and negatively-charged nonmetal anions are held together by electrostatic forces, or “opposites attract.”
- How to find charge based on an ion’s location on the periodic table: Metals lose their valence electrons to become positively charged, and nonmetals gain valence electrons to achieve a full octet.
- That ionic bonds can hold together multiple ions, but the overall charge must add up to zero. You should also be able to figure out how many of each type of ion is required, based on their charge.
Learning Activity
Content contributors: Emma Moulton, Eli Levine, Emily Zhang