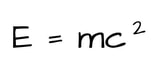The Atom
What is an atom?
Ah, the atom. Especially if you enjoy science, you've probably heard the word before. It's often pronounced "adam," but some people are also good about hitting the "t," as in "at-um". You may have also seen this squiggly, circle-y, orbit-y thing before:
That's an atom! (You'll learn later that this drawing is far from the best way to represent what an atom actually is, but it's a start for a basic model). The atom is so fundamental to science that a drawing like this one of an atom is often used to represent anything vaguely science-y. Atoms are important because they make up every physical thing in the universe. The computer you're using? Made up of atoms. The desk it's sitting on? Made up of atoms. Far away stars? Made up of atoms. Air? Atoms. Water? Atoms. YOU!? You guessed it, atoms.
As a rule: everything (that thing is important: more on this later) is made up of atoms. We often refer to atoms as the "building blocks" or the "fundamental unit" of the universe. Atoms were originally defined by the Greeks as "the smallest unit that can't be divided." In the modern age, because we know more than the Greeks, we have found particles that are smaller than atoms (particles that make up atoms), and we have found ways to split atoms. But, the Greeks were right in saying that an atom is very difficult to split, and that it's probably advisable to not try to split atoms except in very well-controlled environments. For context, this is what happens when you split an atom:
As a rule: everything (that thing is important: more on this later) is made up of atoms. We often refer to atoms as the "building blocks" or the "fundamental unit" of the universe. Atoms were originally defined by the Greeks as "the smallest unit that can't be divided." In the modern age, because we know more than the Greeks, we have found particles that are smaller than atoms (particles that make up atoms), and we have found ways to split atoms. But, the Greeks were right in saying that an atom is very difficult to split, and that it's probably advisable to not try to split atoms except in very well-controlled environments. For context, this is what happens when you split an atom:
(That's an atomic bomb.) Maybe best to stick with the Greeks on this one.
What isn't an atom?
There are, of course, exceptions to every rule. The exception to the rule of "everything is an atom" is that the things that make up atoms, like electrons, protons, and neutrons, are not made up of atoms. The things that make up those electrons, protons, and neutrons are also not atoms. They are called quarks. According to our current best scientific theories, the smallest fundamental thing in the universe is called a string. This theory, called string theory, has not yet been proven, but it explains a lot. It's one of those exciting things that we're still learning about in the universe.
Anything that is not a thing (anything that is not matter) is also not an atom. Other options are space, time, and energy.
Anything that is not a thing (anything that is not matter) is also not an atom. Other options are space, time, and energy.
Matter, Space, Time, and Energy
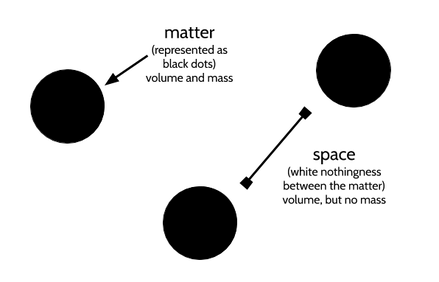
Everything that is a thing is made up of atoms. The proper term for things in the universe is matter. Matter can be touched, weighed, or volumetrically measured. In other words, however small, matter has a volume and a mass: it takes up space and has a weight. A single atom (or a single electron, proton, neutron, or quark) is matter. A ton of bricks is matter. The earth is matter. These things--this matter—can be touched, weighed, or volumetrically measured.
This video provides a good explanation of matter:
This video provides a good explanation of matter:
Space is, in essence, nothingness. It takes up volume but has no mass: It can be measured in terms of distance, but not weight. (It isn't lightweight, like an atom. It has no weight.) Air is made up of matter and a lot of empty space. Water is made up of matter and less empty space. The table you're using is made up of matter and even less empty space. Outer space is made up of A LOT of empty space.
Space is, in essence, nothingness. It takes up volume but has no mass: It can be measured in terms of distance, but not weight. (It isn't lightweight, like an atom. It has no weight.) Air is made up of matter and a lot of empty space. Water is made up of matter and less empty space. The table you're using is made up of matter and even less empty space. Outer space is made up of A LOT of empty space.
Time is very interesting and probably more complicated than you think (thanks to Einstein's theory of relativity). We won't talk about the complexities of time in this class. The basic understanding that you have of it--that time is the progression from one moment to the next, which can be measured in seconds, hours, days, and years--is all you need to know.
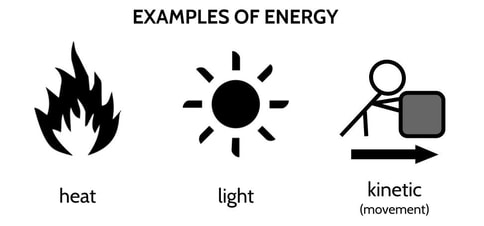
Energy is the potential to do work. If matter describes things, energy describes what we do to those things. Energy comes in many forms, including heat, light, sound, and kinetic energy. Kinetic energy is the energy involved in physically moving things.
Energy is the potential to do work. If matter describes things, energy describes what we do to those things. Energy comes in many forms, including heat, light, sound, and kinetic energy. Kinetic energy is the energy involved in physically moving things.
If you want to be really convinced of how complicated and interesting the universe is, just think of this: matter is things, energy is what we do to those things, and somehow, amazingly, complicatedly, matter can be converted to energy, and energy can be converted to matter. This is explained by the equation:
Where E is Energy, m is mass (of matter), and c is the speed of light. This type of mass to energy conversion is kind of complicated and mostly only relevant when we talk about black holes or splitting apart atoms. Don't worry if this idea of converting matter into energy is complicated and confusing. It's just supposed to blow your mind a bit.
Summary
The universe is very big and very complicated and has lots of interesting stuff going on in it: but, the simplest fundamentals of science really aren't all that difficult to understand. The single, simple, most important takeaway from this lesson? Atoms are tiny particles that take up space, have volume, and make up everything that is a thing (everything that is matter) in the universe.
(Everything... that we know of).
You should understand:
(Everything... that we know of).
You should understand:
- The concept of what an atom is and how it relates to matter.
- The concept of what matter is and how it is different from the other fundamental components of the universe, which are space, time, and energy.
Learning Activity
This is Adam:
Adam is an atom. As you can see, Adam is sad. Adam is having an existential crisis. Adam doesn't understand who he is or why he's so important. Adam doesn't understand that, even though he is just one tiny atom, tiny atoms are absolutely essential to the makeup of the universe.
Adam needs a pep talk. Adam needs to understand what atoms are and why atoms, like Adam, are so important.
Adam needs a pep talk from YOU! In about a paragraph (at least a few sentences), explain to Adam what atoms are and why the universe needs atoms, like Adam. Be as creative as you like in your pep talk, but at least include some basic scientific explanations of what atoms are.
Adam needs a pep talk. Adam needs to understand what atoms are and why atoms, like Adam, are so important.
Adam needs a pep talk from YOU! In about a paragraph (at least a few sentences), explain to Adam what atoms are and why the universe needs atoms, like Adam. Be as creative as you like in your pep talk, but at least include some basic scientific explanations of what atoms are.
Content contributors: Emma Moulton and Emily Zhang