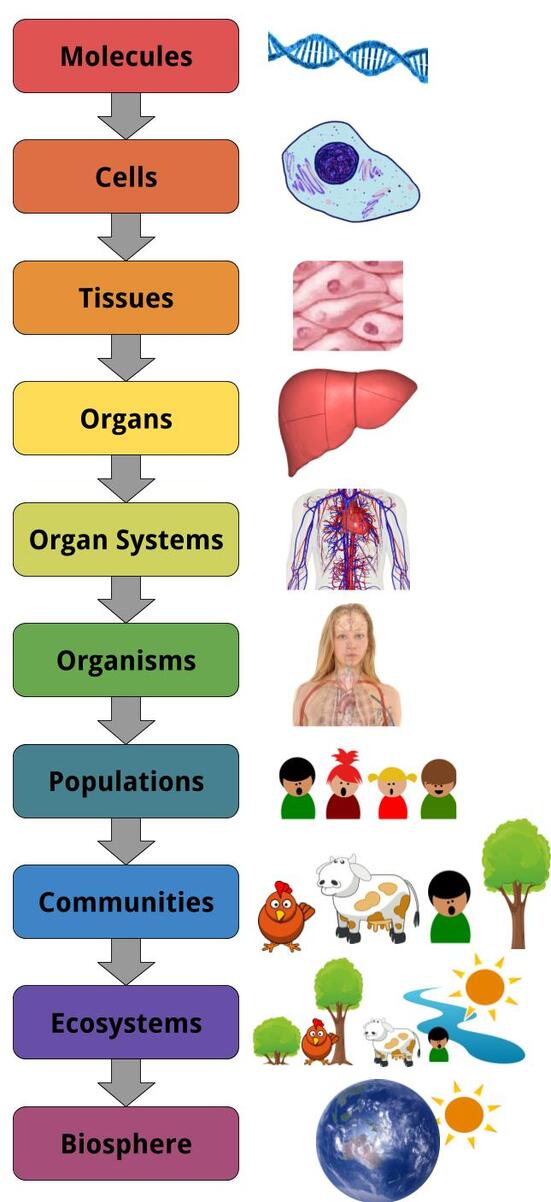
Structural Hierarchy of Living Things
We are all living things – but that does not say much at all about what we are biologically made of. If IKEA wrote on each of their products that ‘matter’ is the sole component, it would be true, but it would not be useful. I would rather know the larger-scale, more specific details like “nuts and bolts” and “metal plates A, B, and C”. With this information, I would have a much better chance of understanding what my Flürgensplürgen was made of and how to put it together.
Similarly, when we consider what living things--organisms—are made of, we can very correctly say that they are made up of matter. But, it is much more useful to have specific terms for how this matter is organized to make up the organism. We also have specific terms for how that organism fits into the grander scheme of life on Earth. These levels of organization range from the very small, like molecules, to the medium-size, like a single plant or animal, to the very large, like an ecosystem,. These levels of organization are:
Similarly, when we consider what living things--organisms—are made of, we can very correctly say that they are made up of matter. But, it is much more useful to have specific terms for how this matter is organized to make up the organism. We also have specific terms for how that organism fits into the grander scheme of life on Earth. These levels of organization range from the very small, like molecules, to the medium-size, like a single plant or animal, to the very large, like an ecosystem,. These levels of organization are:
This type of categorization system is useful the same way that other categorization systems—like defining things as living, non-living, or once living, or classifying elements as metals, nonmetals, and metalloids, or classifying different types of rocks and minerals based on how they were made—are useful. It breaks down a really big, complicated idea into smaller chunks that we can understand better. The difference is that this is an example of vertical organization rather than horizontal or lateral organization. In other words, we’re breaking down the same thing into smaller and smaller chunks, rather than comparing two distinct objects. This should become more clear as you understand the categories better.
Let’s go through them one at a time.
Let’s go through them one at a time.
Molecules
Living things are ultimately made up of molecules (which are made up of atoms, which are made up of quarks, etc., etc.). These molecules range from the very small, like oxygen and water, to the medium-size, to the very large, like proteins and DNA. Some of these molecules are mainly structural (like collagen, which is a protein in your skin and other places), some are purely functional (like trypsin, a protein that breaks down other proteins when you eat), and some are a little of each. Still others don’t really do anything directly except signal to other molecules to start doing their job. Hormones and neurotransmitters are examples of such signalling molecules. We also have molecules that don’t do jobs on their own, but instead help other molecules do their job. Water, vitamins, and the energy molecule ATP are examples of “helper molecules”. The point is, there are a ton of molecules in our bodies doing really important stuff. Really, all life happens at a molecular level, and all the other stuff we’ll talk about is just organization to make those jobs easier.
This video artistically demonstrates life on a molecular level:
This video artistically demonstrates life on a molecular level:
(More specifically, that’s a video of how your immune cells make proteins that help them to get where they need to go.)
We will talk more about some specific biomolecules later on.
Cells
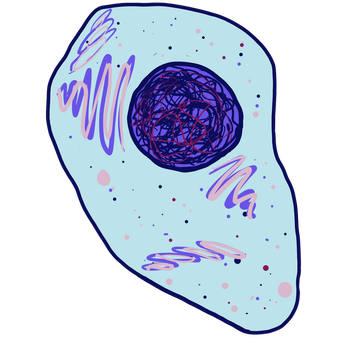
Cells are the basic unit of living things. They are the smallest independent unit that is considered alive. In other words, they do all 7 things that all living things do. They use energy. They grow. They reproduce. Etc. They are made up of molecules. Many organisms—like bacteria and protozoa—are just made up of one cell. In other words, they don’t have tissues or organs or organ systems. Other organisms—like humans and other complex animals—have trillions of cells that are organized into tissues and organs and organ systems (more on this later). You can sort of think of cells as Legos that build people. Except, they’re, like, really, really cool Legos that are alive and have special functions and stuff.
We’ll learn a lot more about cells later. For now, here’s the general idea of what an animal cell looks like:
We’ll learn a lot more about cells later. For now, here’s the general idea of what an animal cell looks like:
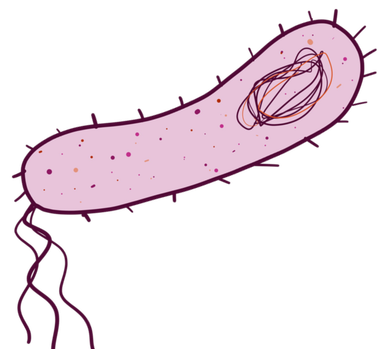
And this is the general idea of what bacteria look like:
Note that the animal cell is a lot more complicated than the bacterial cell. The animal cell is also a lot bigger. These are also both just prototypical structures, which means they’re good examples to give you the general idea, but not all cells in these categories look exactly like these.
Tissues
Tissues are only found in complex multicellular living things, like plants and animals. They are groups of cells and connective tissue, and they have a distinct function. Connective tissue is made of structural proteins that hold cells together, kind of like glue. You may have heard of collagen, elastin, or hyaluronic acid, which are all structural proteins found in connective tissue (that last one is found in a lot of face creams).
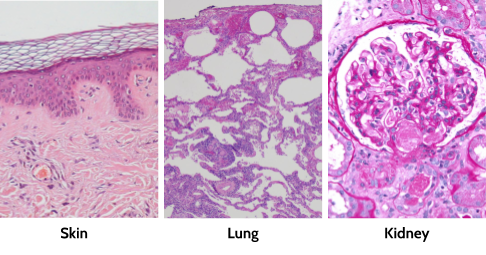
There are generally several types of cells in any given tissue, and each of these cells helps the others to do their job right. It’s like a highly functional workplace. Tissues include a mechanism for delivering fresh nutrients and removing waste; in animals, this is the job of blood vessels. In plants, this is the job of xylem and phloem. Tissues also usually have stem cells, which can turn into some or all of the other cell types in that tissue to replace them if they die.
There are generally several types of cells in any given tissue, and each of these cells helps the others to do their job right. It’s like a highly functional workplace. Tissues include a mechanism for delivering fresh nutrients and removing waste; in animals, this is the job of blood vessels. In plants, this is the job of xylem and phloem. Tissues also usually have stem cells, which can turn into some or all of the other cell types in that tissue to replace them if they die.
|
Tissues under a microscope. They’ve been dyed with a special type of dye to make them pink and purple. Each of the purple dots is the center of a different cell. You can tell the different cell types apart by their shapes and amount of purple dye they take up.
|
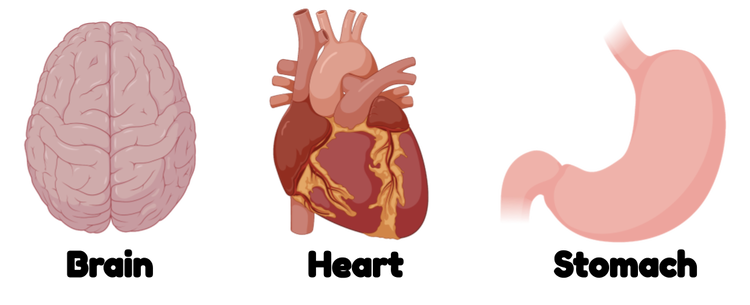
Organs
Organs are larger units of complex living things. They are made up of one or more types of tissue arranged in a pattern or layers. Each organ has a function that helps the living thing survive. For example, your heart pumps blood, your stomach and intestines digest food, and your lungs take in air. Unlike tissues, which kind of blend in with the other tissues in the same organ, each organ is self-contained, or somehow separated from other organs of the body. This is generally by some kind of membrane (thin covering—like the one over the inside of your throat) or sphincter (muscle that closes off openings—like the one that controls when you poop).
Organ Systems
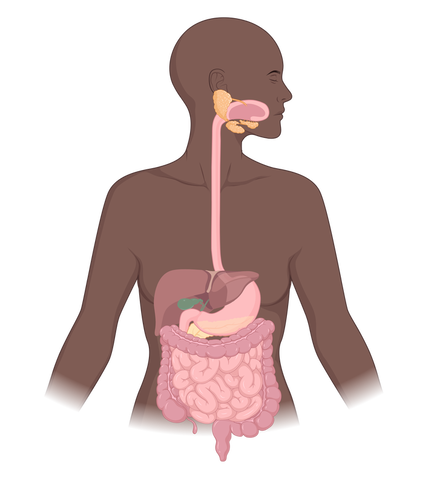
Organ systems are groups of organs that serve a similar function. For example, all the organs of the digestive system are involved in digesting and taking up food. These organs include the mouth, stomach, and intestines.
The organs of the digestive system
Organisms
Organisms are whole, individual plants, animals, bacteria, whatever: a single, complete lifeform. They may be multicellular (like plants and animals) or they may be single-celled (like bacteria).
As a whole unit, organisms meet all of the criteria for life. They are composed of cells. They have different levels of organization, like cells, tissues, and organs. They use energy, such as by digesting food. They grow—in the case of multicellular organisms, this is typically by making new cells (that’s how you grew from a baby!).
Perhaps most importantly, organisms reproduce as a whole unit. We’re not just talking about the cells inside of you dividing—we make babies that look (mostly) like us and grow up to look even more like us. If a colony of bacteria keeps dividing, it just becomes a bigger colony of bacteria—it has no way of making a copy of that whole colony at once. So, even though a single bacteria is alive, colony of bacteria is not its own living organism. But, a single human cell is alive, and so is a human. In short, an organism must meet all of the criteria for life as a whole unit—it’s not just made of living things, but, rather, it is living.
As a whole unit, organisms meet all of the criteria for life. They are composed of cells. They have different levels of organization, like cells, tissues, and organs. They use energy, such as by digesting food. They grow—in the case of multicellular organisms, this is typically by making new cells (that’s how you grew from a baby!).
Perhaps most importantly, organisms reproduce as a whole unit. We’re not just talking about the cells inside of you dividing—we make babies that look (mostly) like us and grow up to look even more like us. If a colony of bacteria keeps dividing, it just becomes a bigger colony of bacteria—it has no way of making a copy of that whole colony at once. So, even though a single bacteria is alive, colony of bacteria is not its own living organism. But, a single human cell is alive, and so is a human. In short, an organism must meet all of the criteria for life as a whole unit—it’s not just made of living things, but, rather, it is living.
A single pant, a single animal, and a single bacterium (“bacteria” is plural for “bacterium”) are all examples of organisms.
Populations
A population is a group of multiple organisms of the same species: a colony of bacteria, your family (pets excluded), a pack of dogs, a flock of seagulls, a murder of crows, a parliament of owls, a whatever-the-group-noun-for-octopus-is of octopi, and so on. The organisms can support each other, compete with each other, or both. But, unlike an organism, it is not quite alive—it doesn’t reproduce as a whole unit. It is just a group of other living things, all of which are the same species.
Each dog is a separate organism, and together these three dogs are an example of a population.
Communities
A community is a group of multiple organisms of different species: plants, animals, bacteria, fungi—anything. A group of plants, animals, bacteria, and fungi on farm is an example. A group of plants, animals, bacteria, and fungi in a forest is another. Your family and all of your favorite pets is a third. Like in a population, the different members of a community may help each other or compete with each other, and it’s usually a little of both.
|
People, dogs, and plants are all part of the same community, but non-living things are not part of that community. (It would actually even be more accurate to say that the clothes they are wearing are not a part of their community, but let’s go ahead and leave them clothed for our purposes).
|
Ecosystems
An ecosystem is a community—a group of lots of different living things—plus all of the non-living things that make life possible. These non-living, life-allowing things are called abiotic factors (“bio-” meaning life, like “biology,” and “a-” meaning “without,” like “atypical”, “asocial,” or “acellular”). Abiotic factors include air, water, sunlight, rocks, minerals, and nutrients. Even if something only very indirectly helps something to survive, it is considered an abiotic factor.
|
People, dogs, plants, and all of the abiotic factors that help them live—like soil, rocks, water, sunlight, and clothing—are all part of the same ecosystem.
|
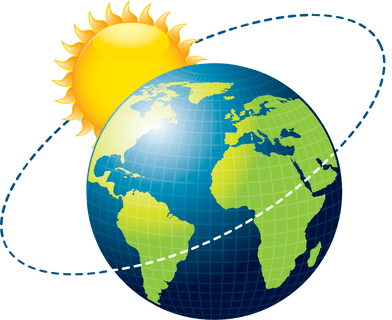
Biosphere
A biosphere encompasses the relationships among all living and nonliving things. In short, it is all of Earth (plus the sun).
Summary
Life is a big, complicated idea. In order to understand big, complicated ideas, we break them down into littler, simpler ideas. Life can be categorized a lot of ways: for example, as plants, animals, and bacteria, or as things with brains and things without brains, or as things that reproduce sexually or asexually, or as things that like the color purple and things that don’t. We’ll talk about some more examples of horizontal categories like these ones later on in this course. For now, it’s important to understand how a single organism can be broken down into many systems or put into larger systems as part of vertical categorization.
You should understand:
This video provides a helpful review of the structural hierarchy of living things. You’ll notice, again, that they list categories that are just a little different than ours (they don’t include molecules as its own category, and they do include something called a biome which is in between an ecosystem and a biosphere). This should be another reminder that any classification scheme is imperfect:
You should understand:
- That organisms have organ systems, which are made up of organs, which are made up of tissues, which are made up of cells, which are made up of molecules.
- That organisms are part of populations, which are part of communities, which are part of ecosystems, which are part of our biosphere.
- What each level of organization includes, and the key features that make it different from other categories. For example, tissues aren’t just groups of cells, they’re groups of cells with connective tissue and a blood supply. Organisms aren’t just groups of cells, they’re groups of organ systems (which have cells) that together make something that meets all of the criteria for life. And so on.
This video provides a helpful review of the structural hierarchy of living things. You’ll notice, again, that they list categories that are just a little different than ours (they don’t include molecules as its own category, and they do include something called a biome which is in between an ecosystem and a biosphere). This should be another reminder that any classification scheme is imperfect:
Learning Activity
Contributors: Alan Li, Emma Moulton
Some images made using biorender.com
Some images made using biorender.com