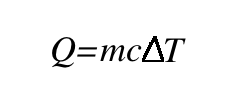Heat
When we speak in normal daily conversation, we often talk about heat and temperature as if they were the same thing. But, in fact, there is an important difference. As mentioned in the previous section, temperature is a measure of how much kinetic energy particles have. Higher temperatures mean faster-moving particles, and lower temperatures mean slower-moving particles. Everything has a temperature, and the temperature (in Kelvin) can never be 0, because particles are always moving. Kelvin temperatures also can't be negative—this just wouldn't make any sense (it would be like saying "I am negative 3 feet tall"). Stuff just is a temperature—it doesn't matter where it came from or where it's going. We call this a state function.
Heat, on the other hand, is a path function. It relies on a change in temperature, not just temperature itself. When we heat something up, we are changing the temperature to a higher one. On a physical level, this means that energy is moving—it is being transferred from one thing to another. So, let's look at some ways that energy can move.
Heat, on the other hand, is a path function. It relies on a change in temperature, not just temperature itself. When we heat something up, we are changing the temperature to a higher one. On a physical level, this means that energy is moving—it is being transferred from one thing to another. So, let's look at some ways that energy can move.
Exothermic: System to Surroundings
In order to talk about energy transfer, we must first talk about where it's going. We can very generally define the areas energy can exist as the system and the surroundings.
The system is the thing we're looking at. If we're talking about an ice cube melting, it's the ice cube. If we're talking about dry ice sublimating, it's the dry ice. If we're talking about a ball of steel heating up, it's the ball of steel.
The surroundings include everything but the system that will interact with the system. This could be the surrounding air, the countertop, the glassware, a bucket of water, or really anything else in the general proximity of the system.
Take a moment to understand what a system and its surroundings are. Come up with some examples that I haven't already listed.
Great. Now we can talk about exothermic, endothermic, and isothermic heat transfers. First up: exothermic.
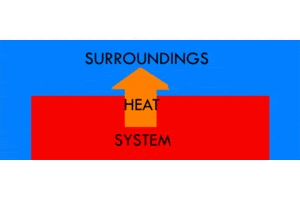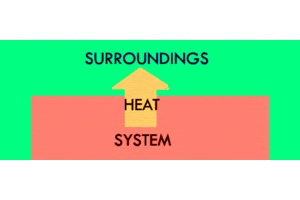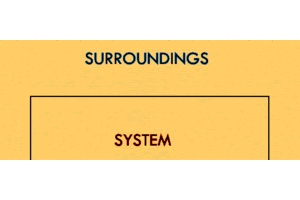
An exothermic reaction is when the system gives off heat to its surroundings. “Exo” means “outside”—so, in an exothermic reaction, we’re making the “outside” surroundings warmer. The temperature of the system will go down, and the temperature of the surroundings will go up. A firework exploding is an example of an exothermic reaction. This should be pretty intuitive: we are producing heat by releasing energy from chemical bonds, and, as a result, the environment heats up. Releasing heat. Exothermic.
An ice cube freezing is another example of an exothermic reaction. This might seem a little less intuitive, but, if we think about it, it’s very similar to the explosion. The system gets colder, so the environment must heat up. It may be hard to feel the change in the temperature of the environment, on the scale of, say, an ice cube freezing in a freezer, because the space in the freezer is so much bigger than the ice cube. But, have you ever felt the back of a freezer on the outside? It’s super warm! The system (the freezer) is getting colder, so the surroundings (the outside back of the freezer) must be getting warmer. And, if the surroundings are getting warmer—if we’re releasing heat—the reaction must be exothermic.
The system is the thing we're looking at. If we're talking about an ice cube melting, it's the ice cube. If we're talking about dry ice sublimating, it's the dry ice. If we're talking about a ball of steel heating up, it's the ball of steel.
The surroundings include everything but the system that will interact with the system. This could be the surrounding air, the countertop, the glassware, a bucket of water, or really anything else in the general proximity of the system.
Take a moment to understand what a system and its surroundings are. Come up with some examples that I haven't already listed.
Great. Now we can talk about exothermic, endothermic, and isothermic heat transfers. First up: exothermic.
An exothermic reaction is when the system gives off heat to its surroundings. “Exo” means “outside”—so, in an exothermic reaction, we’re making the “outside” surroundings warmer. The temperature of the system will go down, and the temperature of the surroundings will go up. A firework exploding is an example of an exothermic reaction. This should be pretty intuitive: we are producing heat by releasing energy from chemical bonds, and, as a result, the environment heats up. Releasing heat. Exothermic.
An ice cube freezing is another example of an exothermic reaction. This might seem a little less intuitive, but, if we think about it, it’s very similar to the explosion. The system gets colder, so the environment must heat up. It may be hard to feel the change in the temperature of the environment, on the scale of, say, an ice cube freezing in a freezer, because the space in the freezer is so much bigger than the ice cube. But, have you ever felt the back of a freezer on the outside? It’s super warm! The system (the freezer) is getting colder, so the surroundings (the outside back of the freezer) must be getting warmer. And, if the surroundings are getting warmer—if we’re releasing heat—the reaction must be exothermic.
Endothermic: Surroundings to System
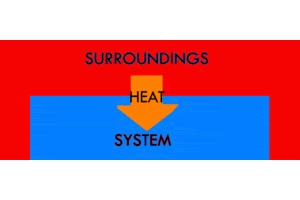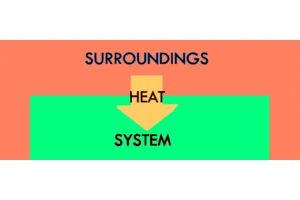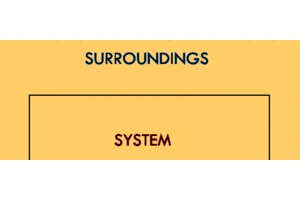
Endothermic is the opposite of exothermic. "Endo" means “inside”: you can remember this because "en" and "in" sound similar. So, in an endothermic reaction, heat moves from the outside to the inside, surroundings to the system. The temperature of the system increases, and the temperature of the surroundings decreases. An ice cube melting is an example of an endothermic reaction, as is water boiling. These things require that you take heat from the environment to make the system warmer.
This video gives a great explanation of the difference between exothermic and endothermic reaction:
Isothermic: Back and Forth
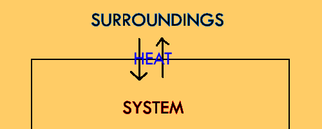
In an isothermic reaction, no net heat moves between the system and the surroundings. According to the laws of nature, heat will always be moving between the system and the surroundings simply because particles can't help but bump into one another. But, if the system and the surroundings have the same amount of heat potential energy (heat that hasn't moved yet) associated with them, then there will be no driving force for heat to move in one direction or the other. So, overall they will stay at roughly the same temperature (actually the same heat potential energy, but they're closely related), even though heat is moving back and forth.
I probably just confused you, especially if you've never heard of potential energy or conservation of energy before, but don't worry too much about the explanation for now. Just know that heat is always moving back and forth, even if nothing is getting hotter or colder. This is called an isothermic reaction. “Iso” means “same.”
I probably just confused you, especially if you've never heard of potential energy or conservation of energy before, but don't worry too much about the explanation for now. Just know that heat is always moving back and forth, even if nothing is getting hotter or colder. This is called an isothermic reaction. “Iso” means “same.”
Calculating Heat
The concept of heat is the most important thing that you should focus on understanding at this level—heat moves between a system and its surroundings in exothermic, endothermic, or isothermic processes. However, it is also important to note that we can calculate heat based on the equation below. You will learn more about the different ways that you can use this equation in higher level classes, but I'll introduce the basics now.
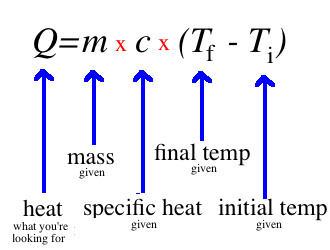
You don’t need to know where this equation comes from to use it, but you may find it helpful to understand. Unlike temperature, which can be easily measured with a thermometer, we don't really have a great way of measuring heat. Instead, we calculate it based on temperature. Recall that heat relies on a change in temperature. So, in order to calculate heat, we will need a starting (initial) temperature and an ending (final) temperature. Subtracting these will give us the change. Heat also depends on the mass of something—how much it weighs. This is easy to understand if you think about boiling a pot of water. It is going to take a long time to boil a big pot of water, but only a few seconds to boil a small drop. Lastly, the heat will depend on what you're heating up. For instance, alcohol boils a lot faster than water. This is represented by the letter "c" and is a set number based on what you are heating up. This can be calculated using very advanced physics, but you can also just look up an accepted value in a book or on the internet. For our purposes, it will always be given to you. So, putting the temperature change, the mass, and "c" together, we come up with this equation. So, just subtract the initial temperature from the final temperature and multiply this number by the mass and the specific heat, and you've found the heat of the system!
So, just subtract the initial temperature from the final temperature and multiply this number by the mass and the specific heat, and you've found the heat of the system!
FYI, this equation can also be written as:
So, just subtract the initial temperature from the final temperature and multiply this number by the mass and the specific heat, and you've found the heat of the system!
FYI, this equation can also be written as:
This video gives a great tutorial on using the equation for heat.
Summary
You should understand:
- The difference between heat (the transfer of energy, a path function) and temperature (average kinetic energy, a state function)
- How to identify whether a reaction is exothermic, endothermic, or isothermic, depending on the change in temperature of the system and surroundings.
- How to calculate heat from a given mass, specific heat constant, and change in temperature, using the equation q = mcΔT.
Learning Activity
Content contributors: Emma Moulton