Modeling Ionic Compounds
We've already discussed what types of atoms will attract each other. We've also talked a little bit about why atoms come together--because of electrostatics and stability. However, we still don’t know exactly how the atoms actually combine to form molecules: Or, in other words, what compounds and molecules look like. You can think of atoms as kind of like LEGOs that adhere to form compounds, molecules, and everything that surrounds us. (This, of course, is over-simplified and inaccurate. But, it’s useful enough for our level).
Way back when we made atomic symbols and Bohr Models, we learned that scientists like to use pictures to show people things instead of trying to explain it to them. Remember this guy?
Way back when we made atomic symbols and Bohr Models, we learned that scientists like to use pictures to show people things instead of trying to explain it to them. Remember this guy?
This is the "car" we might come up with if we had never seen a car and someone just told us that it has an engine and a place for people to sit, described a steering wheel, and explained how it gets you places faster than walking would. So, to avoid confusion, we make pictures. This lesson is basically a scientific art class with atoms.
Modeling Ionic Compounds
As you've learned, there are two main types of chemical bonding: ionic bonding and covalent bonding. You should know that an ionic bond is between a metal and a nonmetal, and should understand that it involves charged ions. It involves a nonmetal, which basically steals an electron (or two or three) from a metal. This makes the nonmetal negatively charged and the metal positively charged. Since opposites attract, they stick together in an ionic bond. But, these are words, and scientists like pictures. So let's learn to draw pictures!
The Octet Rule / I Wanna Be A Noble Gas
Remember when I told you the octet rule was super important? Well, it's about to become even more important. So, if you don't remember it, go back and review before continuing this lesson.
Brief reminder: 🎵I wanna be a noble gas, like neon and helium… 🎵(Side note: do people still watch Pokemon?)
Brief reminder: 🎵I wanna be a noble gas, like neon and helium… 🎵(Side note: do people still watch Pokemon?)
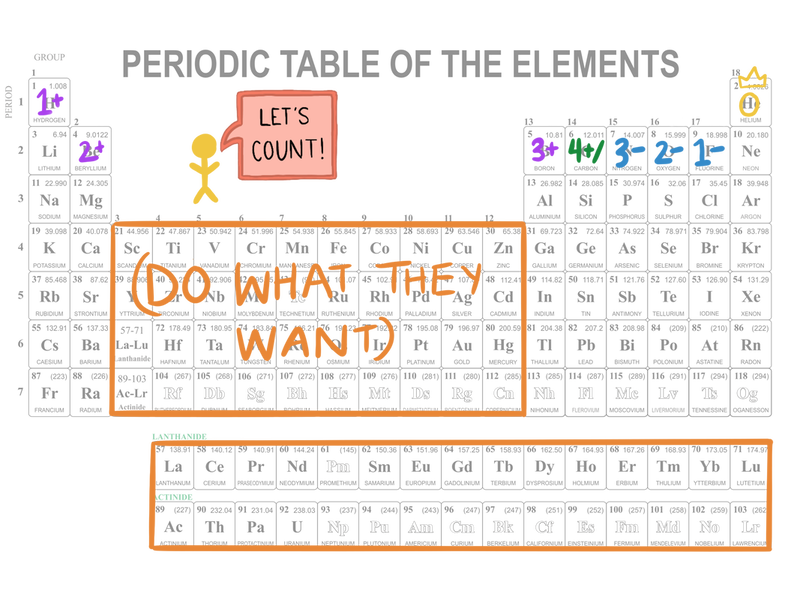
Determining the charge on a metal or nonmetal ion, based on its position on the Periodic Table. More details can be found in Chemical Nomenclature: Ionic Compounds. (Side note: Transition metals don’t really *just* do whatever they want, but it sure seems like it sometimes, so we won’t deal with them here).
Bohr models
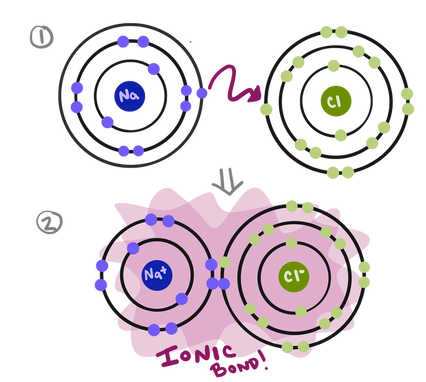
One way to model ionic compounds is using Bohr models similar to the ones I drew when we were first learning about Ionic Bonding. But, drawing out the *whooooole* Bohr model takes up a lot of space and can be kind of annoying, so, instead, we make simplified little models called Lewis dot structures that have only the atomic symbol and the valence electrons.
Modeling ionic compounds with Bohr Models. It paints a picture, but it can be simplified a lot.
Lewis Dot Structures
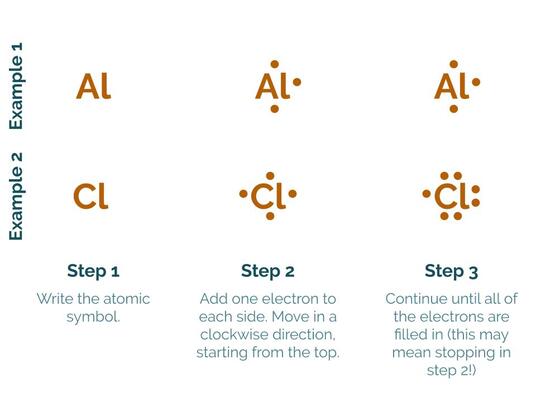
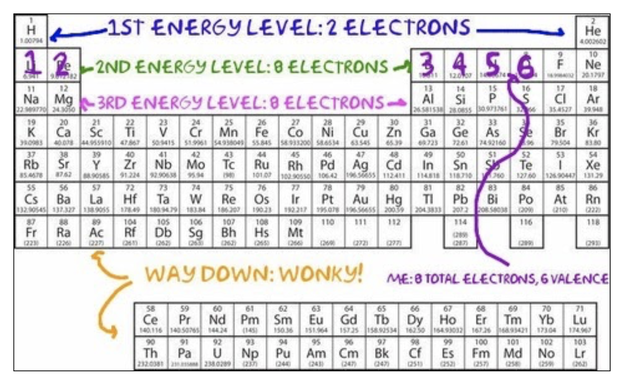
As it happens, Lewis dot structures were invented by a guy named Lewis (how convenient that he managed to invent something with the same name as him!). They’re extremely simple to read: each dot represents a valence electron, and each atom is denoted with its atomic symbol. To draw them, start with the atomic symbol in the middle, and just keep putting dots in a clockwise circle until all of the valence electrons are represented by a dot. To figure out how many are enough, just count the boxes on the Periodic table like we did with Bohr models. Remember, the number of valance electrons is the number of spaces that an element is from the first column on the periodic table:
Modeling ionic compounds with Lewis Dot Structures
Determining the number of valence electrons using the Periodic Table
Modeling Ionic Compounds with Lewis Dot Structures
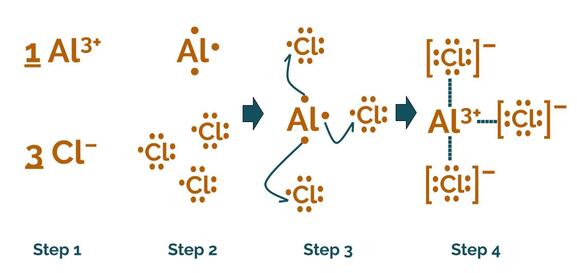
Modeling an ionic compound (and the chemical reaction that makes it) with Lewis dot structures is almost as easy as drawing a Lewis dot structure. It's just 4 easy steps:
Modeling an ionic reaction with Lewis dot structures
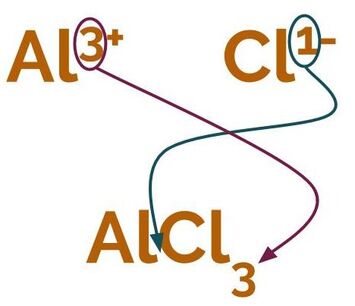
- Write out the relevant ions with their charges and decide how many of each ion is required to make a balanced equation. Remember, all charges should add up to zero. One easy way to do this is to say that the charges of one ion should be the subscript on the other (simplified to the lowest common denominator). So, Al³⁺ and Cl⁻ would make AlCl₃, and Al³⁺ and O²⁻ would make Al₂O₃. We sometimes call this the criss-cross rule.
- Draw Lewis Dot Structures for each atom in the molecule. If there is more than one instance of each atom, make sure to draw all of them! This is what we learned how to do in the first part of the lesson.
- Transfer electrons from atom to atom (arrows). Make sure each metal gives away all of its valence electrons, and that each nonmetal ends up with a full valence shell of 8 electrons (or only 2 for hydrogen, the exception to the octet rule).
- Draw the new Lewis dot structures. Be sure to indicate the charge of the atom. With larger structures like these, we do this by putting the structure in brackets and putting the charge outside the brackets, as shown in the picture. You can show electrostatic attraction between the newly formed ions with a dotted line.
Using the criss-cross rule
Drawing Lewis dot structures and modeling ionic compounds isn't too complicated, but it is important to know these steps in order to understand our next step: modeling covalent compounds. As always, the more you practice, the easier it gets.
Summary
You should know:
- How to draw Lewis dot structures for single atoms/ions, by figuring out the number of valence electrons.
- How to model ionic compounds using Lewis dot structures, by showing how valence electrons get transferred and how the resulting charged ions are held together by electrostatic interactions.
Learning Activity
| Learning Activity Modeling Ionic Compounds.pdf |
This is one of those things that you’ll never really learn unless you do it—so, this activity will give you the chance to do it!
For the following, you may draw out your answers in any format you like, but it is probably easiest to do them on a piece of paper.
For the following atoms or ions, please draw the correct Lewis dot structure.
Model the following ionic compounds and the reaction that produced them. Outline all 4 steps as shown in the lesson.
For the following, you may draw out your answers in any format you like, but it is probably easiest to do them on a piece of paper.
For the following atoms or ions, please draw the correct Lewis dot structure.
- Magnesium
- Bromine (note: Ignore the transition metals. This should look very similar to chlorine.)
- Bromide (note: Ignore the transition metals. This should look very similar to chloride.)
- Carbon
- Silicon
- Phosphorus
- Neon
- Nitrogen
- Oxygen
- Hydrogen
Model the following ionic compounds and the reaction that produced them. Outline all 4 steps as shown in the lesson.
- Calcium fluoride
- Lithium oxide
Content contributors: Eli Levine, Nancy Jiang, Emma Moulton